Application of NPE in the assessment of a patent ductus arteriosus
- PMID: 30072803
- PMCID: PMC6257219
- DOI: 10.1038/s41390-018-0077-x
Application of NPE in the assessment of a patent ductus arteriosus
Abstract
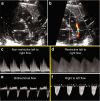
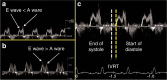
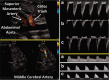
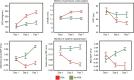
In many preterm infants, the ductus arteriosus remains patent beyond the first few days of life. This prolonged patency is associated with numerous adverse outcomes, but the extent to which these adverse outcomes are attributable to the hemodynamic consequences of ductal patency, if at all, has not been established. Different treatment strategies have failed to improve short-term outcomes, with a paucity of data on the correct diagnostic and pathophysiological assessment of the patent ductus arteriosus (PDA) in association with long-term outcomes. Echocardiography is the selected method of choice for detecting a PDA, assessing the impact on the preterm circulation and monitoring treatment response. PDA in a preterm infant can result in pulmonary overcirculation and systemic hypoperfusion, Therefore, echocardiographic assessment should include evaluation of PDA characteristics, indices of pulmonary overcirculation with left heart loading conditions, and indices of systemic hypoperfusion. In this review, we provide an evidence-based overview of the current and emerging ultrasound measurements available to identify and monitor a PDA in the preterm infant. We offer indications and limitations for using Neonatologist Performed Echocardiography to optimize the management of a neonate with a PDA.
Conflict of interest statement
A.E.K. is in receipt of an Irish Health Research Board Clinical Trials Network Grant (HRB CTN 2014-10) and an EU FP7/2007-2013 grant (agreement no. 260777, The HIP Trial). A.G. owned equity in Neonatal Echo Skills and has received grant support from the American Heart Association. E.D. received lecture fees and consulting fees from Chiesi Pharmaceutical. E.N. received grant support from Research Council of Norway and Vestfold Hospital Trust. K.B. received lecture fees from Chiesi Pharmaceutical. M.B. holds a patent “Thermal shield for the newborn baby”. S.R. received lecture fees for Phillips Ultrasound and GE Ultrasound. W.P.B. has received grant support from The Netherlands Organization for Health and Development (ZonMw; grant number 843002608). Z.M. has received lecture fees from Chiesi Pharmaceutical. The remaining authors declared no competing interests.
Figures


Comment in
-
Targeted neonatal echocardiography in the United States of America: the contemporary perspective and challenges to implementation.Pediatr Res. 2019 Jun;85(7):919-921. doi: 10.1038/s41390-019-0338-3. Epub 2019 Feb 18. Pediatr Res. 2019. PMID: 30776791 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

