Fiber Supplements Derived From Sugarcane Stem, Wheat Dextrin and Psyllium Husk Have Different In Vitro Effects on the Human Gut Microbiota
- PMID: 30072976
- PMCID: PMC6060387
- DOI: 10.3389/fmicb.2018.01618
Fiber Supplements Derived From Sugarcane Stem, Wheat Dextrin and Psyllium Husk Have Different In Vitro Effects on the Human Gut Microbiota
Abstract
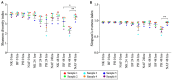
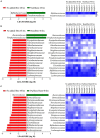
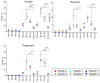
There is growing public interest in the use of fiber supplements as a way of increasing dietary fiber intake and potentially improving the gut microbiota composition and digestive health. However, currently there is limited research into the effects of commercially available fiber supplements on the gut microbiota. Here we used an in vitro human digestive and gut microbiota model system to investigate the effect of three commercial fiber products; NutriKane™, Benefiber® and Psyllium husk (Macro) on the adult gut microbiota. The 16S rRNA gene amplicon sequencing results showed dramatic fiber-dependent changes in the gut microbiota structure and composition. Specific bacterial OTUs within the families Bacteroidaceae, Porphyromonadaceae, Ruminococcaceae, Lachnospiraceae, and Bifidobacteriaceae showed an increase in the relative abundances in the presence of one or more fiber product(s), while Enterobacteriaceae and Pseudomonadaceae showed a reduction in the relative abundances upon addition of all fiber treatments compared to the no added fiber control. Fiber-specific increases in SCFA concentrations showed correlation with the relative abundance of potential SCFA-producing gut bacteria. The chemical composition, antioxidant potential and polyphenolic content profiles of each fiber product were determined and found to be highly variable. Observed product-specific variations could be linked to differences in the chemical composition of the fiber products. The general nature of the fiber-dependent impact was relatively consistent across the individuals, which may demonstrate the potential of the products to alter the gut microbiota in a similar, and predictable direction, despite variability in the starting composition of the individual gut microbiota.
Keywords: 16S rRNA gene; dietary fiber supplementation; gut microbiota; in vitro gut models; polyphenols; short chain fatty acids.
Figures


References
-
- Benzie I. F., Strain J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 239, 70–76. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

