Natural Killer Cells Exhibit a Peculiar Phenotypic Profile in Systemic Sclerosis and Are Potent Inducers of Endothelial Microparticles Release
- PMID: 30072999
- PMCID: PMC6058015
- DOI: 10.3389/fimmu.2018.01665
Natural Killer Cells Exhibit a Peculiar Phenotypic Profile in Systemic Sclerosis and Are Potent Inducers of Endothelial Microparticles Release
Abstract
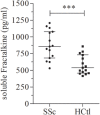
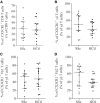
The pathophysiology of systemic sclerosis (SSc) involves early endothelial and immune activation, both preceding the onset of fibrosis. We previously identified soluble fractalkine and circulating endothelial microparticles (EMPs) as biomarkers of endothelial inflammatory activation in SSc. Fractalkine plays a dual role as a membrane-bound adhesion molecule expressed in inflamed endothelial cells (ECs) and as a chemokine involved in the recruitment, transmigration, and cytotoxic activation of immune cells that express CX3CR1, the receptor of fractalkine, namely CD8 and γδ T cells and natural killer (NK) cells. We aimed to quantify circulating cytotoxic immune cells and their expression of CX3CR1. We further investigated the expression profile of NK cells chemokine receptors and activation markers and the potential of NK cells to induce EC activation in SSc. We performed a monocentric study (NCT 02636127) enrolling 15 SSc patients [15 females, median age of 55 years (39-63), 11 limited cutaneous form and 4 diffuse] and 15 healthy controls. Serum fractalkine levels were significantly increased in SSc patients. Circulating CD8 T cells numbers were decreased in SSc patients with no difference in their CX3CR1 expression. Circulating γδ T cells and NK cells numbers were preserved. CX3CR1 expression in CD8 and γδ T cells did not differ between SSc patients and controls. The percentage and level of CX3CR1 expression in NK cells were significantly lowered in SSc patients. Percentages of CXCR4, NKG2D, CD69-expressing NK cells, and their expression levels were decreased in NK cells. Conversely, CD16 level expression and percentages of CD16+ NK cells were preserved. The exposure of human microvascular dermic EC line (HMVEC-d) to peripheral blood mononuclear cells resulted in similar NK cells degranulation activity in SSc patients and controls. We further showed that NK cells purified from the blood of SSc patients induced enhanced release of EMPs than NK cells from controls. This study evidenced a peculiar NK cells phenotype in SSc characterized by decreased chemokine and activation receptors expression, that might reflect NK cells involvement in the pathogenic process. It also highlighted the role of NK cells as a potent mechanism inducing endothelial activation through enhanced EMPs release.
Keywords: CX3CR1; endothelial microparticles; fractalkine; natural killer cells; systemic sclerosis.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

