Synthesis, Structure, DNA Interaction, and SOD Activity of Three Nickel(II) Complexes Containing L-Phenylalanine Schiff Base and 1,10-Phenanthroline
- PMID: 30073020
- PMCID: PMC6057355
- DOI: 10.1155/2018/8478152
Synthesis, Structure, DNA Interaction, and SOD Activity of Three Nickel(II) Complexes Containing L-Phenylalanine Schiff Base and 1,10-Phenanthroline
Abstract
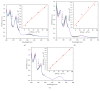
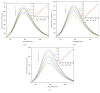
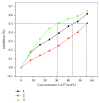
Three hexacoordinated octahedral nickel(II) complexes, [Ni(sal-L-phe)(phen)(CH3OH)]·CH3OH (1), [Ni(naph-L-phe)(phen)(CH3OH)] (2), and [Ni(o-van-L-phe)(phen)(CH3OH)]·5CH3OH (3) (sal-L-phe = a Schiff base derived from salicylaldehyde and L-phenylalanine, naph-L-phe = a Schiff base derived from 2-hydroxy-1-naphthaldehyde and L-phenylalanine, o-van-L-phe = a Schiff base derived from o-vanillin and L-phenylalanine, and phen = 1,10-phenanthroline), have been synthesized and characterized by elemental analysis, IR spectra, and single-crystal X-ray diffraction. The interactions of these complexes with CT-DNA were studied by UV-Vis absorption spectroscopy, fluorescence spectroscopy, circular dichroism spectroscopy, and viscosity measurements. The binding constant (Kb) values of 1.82 × 104 M-1 for 1, 1.96 × 104 M-1 for 2, and 2.02 × 104 M-1 for 3 suggest that each of these complexes could bind with DNA in a moderate intercalative mode. Complex 3 exhibited a stronger interaction with CT-DNA than complexes 1 and 2. In addition, the superoxide scavenging activity of these complexes was investigated by the nitrotetrazolium blue chloride (NBT) light reduction method, and the results showed that they exhibited a significant superoxide scavenging activity with the IC50 values of 4.4 × 10-5 M for complex 1, 5.6 × 10-5 M for complex 2, and 3.1 × 10-5 M for complex 3, respectively.
Figures








References
-
- Liu Y. J., Zeng C. H. Synthesis and DNA interaction studies of ruthenium(II) complexes with isatino[1,2-b]-1,4,8,9-tetraazatriphenylene as an intercalative ligand. Transition Metal Chemistry. 2009;34(4):455–462. doi: 10.1007/s11243-009-9216-x. - DOI
-
- Liu H., Li L., Guo Q., Dong J., Li J. Synthesis, crystal structure, DNA and BSA binding properties of a chromium(III) complex with L-glycine Schiff base and 1,10-phenanthroline. Transition Metal Chemistry. 2013;38(4):441–448. doi: 10.1007/s11243-013-9709-5. - DOI
-
- Genin M. J., Biles C., Keiser B. J., et al. Novel 1,5-diphenylpyrazole nonnucleoside HIV-1 reverse transcriptase inhibitors with enhanced activity versus the delavirdine-resistant P236L mutant: lead identification and SAR of 3- and 4-substituted derivatives. Journal of Medicinal Chemistry. 2011;43(5):1034–1040. doi: 10.1021/jm990383f. - DOI - PubMed
-
- Verma M., Pandeya S. N., Singh K. N., Stables J. P. Anticonvulsant activity of Schiff bases of isatin derivatives. Acta Pharmaceutica. 2004;54(1):49–56. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

