Placebo Effects on the Neurologic Pain Signature: A Meta-analysis of Individual Participant Functional Magnetic Resonance Imaging Data
- PMID: 30073258
- PMCID: PMC6248115
- DOI: 10.1001/jamaneurol.2018.2017
Placebo Effects on the Neurologic Pain Signature: A Meta-analysis of Individual Participant Functional Magnetic Resonance Imaging Data
Abstract
Importance: Placebo effects reduce pain and contribute to clinical analgesia, but after decades of research, it remains unclear whether placebo treatments mainly affect nociceptive processes or other processes associated with pain evaluation.
Objective: We conducted a systematic, participant-level meta-analysis to test the effect of placebo treatments on pain-associated functional neuroimaging responses in the neurologic pain signature (NPS), a multivariate brain pattern tracking nociceptive pain.
Data sources: Medline (PubMed) was searched from inception to May 2015; the search was augmented with results from previous meta-analyses and expert recommendations.
Study selection: Eligible studies were original investigations that were published in English in peer-reviewed journals and that involved functional neuroimaging of the human brain with evoked pain delivered under stimulus intensity-matched placebo and control conditions. The authors of all eligible studies were contacted and asked to provide single-participant data.
Data extraction and synthesis: Data were collected between December 2015 and November 2017 following the Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data guidelines. Results were summarized across participants and studies in a random-effects model.
Main outcomes and measures: The main, a priori outcome was NPS response; pain reports were assessed as a secondary outcome.
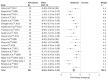
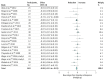
Results: We obtained data from 20 of 28 identified eligible studies, resulting in a total sample size of 603 healthy individuals. The NPS responses to painful stimulation compared with baseline conditions were positive in 575 participants (95.4%), with a very large effect size (g = 2.30 [95% CI, 1.92 to 2.69]), confirming its sensitivity to nociceptive pain in this sample. Placebo treatments showed significant behavioral outcomes on pain ratings in 17 of 20 studies (85%) and in the combined sample (g = -0.66 [95% CI, -0.80 to -0.53]). However, placebo effects on the NPS response were significant in only 3 of 20 studies (15%) and were very small in the combined sample (g = -0.08 [95% CI, -0.15 to -0.01]). Similarly, analyses restricted to studies with low risk of bias (g = -0.07 [95% CI, -0.15 to 0.00]) indicated very small effects, and analyses of just placebo responders (g = -0.22 [95% CI, -0.34 to -0.11]) indicated small effects, as well.
Conclusions and relevance: Placebo treatments have moderate analgesic effects on pain reports. The very small effects on NPS, a validated measure that tracks levels of nociceptive pain, indicate that placebo treatments affect pain via brain mechanisms largely independent of effects on bottom-up nociceptive processing.
Conflict of interest statement
Figures

Comment in
-
Modulation of Nociception in Multiple Brain Systems-The Strain in Pain.JAMA Neurol. 2018 Nov 1;75(11):1309-1310. doi: 10.1001/jamaneurol.2018.1752. JAMA Neurol. 2018. PMID: 30073248 No abstract available.
-
Laterality and Stimulation Bias in Meta-analysis of Placebo Responses-Reply.JAMA Neurol. 2019 Jul 1;76(7):870. doi: 10.1001/jamaneurol.2019.1232. JAMA Neurol. 2019. PMID: 31107506 No abstract available.
-
Laterality and Stimulation Bias in Meta-analysis of Placebo Responses.JAMA Neurol. 2019 Jul 1;76(7):869-870. doi: 10.1001/jamaneurol.2019.1229. JAMA Neurol. 2019. PMID: 31107522 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

