Safety and Efficacy of Atorvastatin for Chronic Subdural Hematoma in Chinese Patients: A Randomized ClinicalTrial
- PMID: 30073290
- PMCID: PMC6248109
- DOI: 10.1001/jamaneurol.2018.2030
Safety and Efficacy of Atorvastatin for Chronic Subdural Hematoma in Chinese Patients: A Randomized ClinicalTrial
Abstract
Importance: Chronic subdural hematoma (CSDH) is a trauma-associated condition commonly found in elderly patients. Surgery is currently the treatment of choice, but it carries a significant risk of recurrence and death. Nonsurgical treatments remain limited and ineffective. Our recent studies suggest that atorvastatin reduces hematomas and improves the clinical outcomes of patients with CSDH.
Objective: To investigate the safety and therapeutic efficacy of atorvastatin to nonsurgically treat patients with CSDH.
Design, setting, and participants: The Effect of Atorvastatin on Chronic Subdural Hematoma (ATOCH) randomized, placebo-controlled, double-blind phase II clinical trial was conducted in multiple centers in China from February 2014 to November 2015. For this trial, we approached 254 patients with CSDH who received a diagnosis via a computed tomography scan; of these, 200 (78.7%) were enrolled because 23 patients (9.1%) refused to participate and 31 (12.2%) were disqualified.
Interventions: Patients were randomly assigned to receive either 20 mg of atorvastatin or placebo daily for 8 weeks and were followed up for an additional 16 weeks.
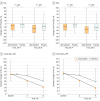
Main outcomes and measures: The primary outcome was change in hematoma volume (HV) by computed tomography after 8 weeks of treatment. The secondary outcomes included HV measured at the 4th, 12th, and 24th weeks and neurological function that was evaluated using the Markwalder grading scale/Glasgow Coma Scale and the Barthel Index at the 8th week.
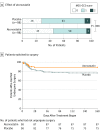
Results: One hundred ninety-six patients received treatment (169 men [86.2%]; median [SD] age, 63.6 [14.2] years). The baseline HV and clinical presentations were similar between patients who were taking atorvastatin (98 [50%]) and the placebo (98 [50%]). After 8 weeks, the HV reduction in patients who were taking atorvastatin was 12.55 mL more than those taking the placebo (95% CI, 0.9-23.9 mL; P = .003). Forty-five patients (45.9%) who were taking atorvastatin significantly improved their neurological function, but only 28 (28.6%) who were taking the placebo did, resulting in an adjusted odds ratio of 1.957 for clinical improvements (95% CI, 1.07-3.58; P = .03). Eleven patients (11.2%) who were taking atorvastatin and 23 (23.5%) who were taking the placebo underwent surgery during the trial for an enlarging hematoma and/or a deteriorating clinical condition (hazard ratio, 0.47; 95% CI, 0.24-0.92; P = .03). No significant adverse events were reported.
Conclusions and relevance: Atorvastatin may be a safe and efficacious nonsurgical alternative for treating patients with CSDH.
Trial registration: ClinicalTrials.gov Identifier: NCT02024373.
Conflict of interest statement
Figures

References
-
- Karibe H, Kameyama M, Kawase M, Hirano T, Kawaguchi T, Tominaga T. Epidemiology of chronic subdural hematomas [article in Japanese]. No Shinkei Geka. 2011;39(12):1149-1153. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

