Unexpected subcellular distribution of a specific isoform of the Coxsackie and adenovirus receptor, CAR-SIV, in human pancreatic beta cells
- PMID: 30074059
- PMCID: PMC6182664
- DOI: 10.1007/s00125-018-4704-1
Unexpected subcellular distribution of a specific isoform of the Coxsackie and adenovirus receptor, CAR-SIV, in human pancreatic beta cells
Abstract
Aims/hypothesis: The Coxsackie and adenovirus receptor (CAR) is a transmembrane cell-adhesion protein that serves as an entry receptor for enteroviruses and may be essential for their ability to infect cells. Since enteroviral infection of beta cells has been implicated as a factor that could contribute to the development of type 1 diabetes, it is often assumed that CAR is displayed on the surface of human beta cells. However, CAR exists as multiple isoforms and it is not known whether all isoforms subserve similar physiological functions. In the present study, we have determined the profile of CAR isoforms present in human beta cells and monitored the subcellular localisation of the principal isoform within the cells.
Methods: Formalin-fixed, paraffin-embedded pancreatic sections from non-diabetic individuals and those with type 1 diabetes were studied. Immunohistochemistry, confocal immunofluorescence, electron microscopy and western blotting with isoform-specific antisera were employed to examine the expression and cellular localisation of the five known CAR isoforms. Isoform-specific qRT-PCR and RNA sequencing (RNAseq) were performed on RNA extracted from isolated human islets.
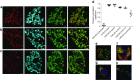
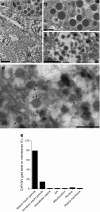
Results: An isoform of CAR with a terminal SIV motif and a unique PDZ-binding domain was expressed at high levels in human beta cells at the protein level. A second isoform, CAR-TVV, was also present. Both forms were readily detected by qRT-PCR and RNAseq analysis in isolated human islets. Immunocytochemical studies indicated that CAR-SIV was the principal isoform in islets and was localised mainly within the cytoplasm of beta cells, rather than at the plasma membrane. Within the cells it displayed a punctate pattern of immunolabelling, consistent with its retention within a specific membrane-bound compartment. Co-immunofluorescence analysis revealed significant co-localisation of CAR-SIV with zinc transporter protein 8 (ZnT8), prohormone convertase 1/3 (PC1/3) and insulin, but not proinsulin. This suggests that CAR-SIV may be resident mainly in the membranes of insulin secretory granules. Immunogold labelling and electron microscopic analysis confirmed that CAR-SIV was localised to dense-core (insulin) secretory granules in human islets, whereas no immunolabelling of the protein was detected on the secretory granules of adjacent exocrine cells. Importantly, CAR-SIV was also found to co-localise with protein interacting with C-kinase 1 (PICK1), a protein recently demonstrated to play a role in insulin granule maturation and trafficking.
Conclusions/interpretation: The SIV isoform of CAR is abundant in human beta cells and is localised mainly to insulin secretory granules, implying that it may be involved in granule trafficking and maturation. We propose that this subcellular localisation of CAR-SIV contributes to the unique sensitivity of human beta cells to enteroviral infection.
Keywords: Beta cells; Coxsackie and adenovirus receptor; Coxsackievirus B; Enterovirus; Insulin granule; Pancreas; Protein interacting with C-kinase 1 (PICK1).
Conflict of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Figures





References
-
- Roivainen M, Rasilainen S, Ylipaasto P, et al. Mechanisms of coxsackievirus-induced damage to human pancreatic beta-cells 1. J Clin Endocrinol Metab. 2000;85:432–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

