A simple decision to move in response to touch reveals basic sensory memory and mechanisms for variable response times
- PMID: 30074236
- PMCID: PMC6292811
- DOI: 10.1113/JP276356
A simple decision to move in response to touch reveals basic sensory memory and mechanisms for variable response times
Abstract
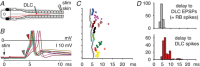
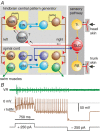
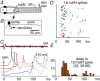
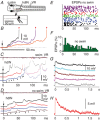
Key points: Short-term working memory and decision-making are usually studied in the cerebral cortex; in many models of simple decision making, sensory signals build slowly and noisily to threshold to initiate a motor response after long, variable delays. When touched, hatchling frog tadpoles decide whether to swim; we define the long and variable delays to swimming and use whole-cell recordings to uncover the neurons and processes responsible. Firing in sensory and sensory pathway neurons is short latency, and too brief and invariant to explain these delays, while recordings from hindbrain reticulospinal neurons controlling swimming reveal a prolonged and variable build-up of synaptic excitation which can reach firing threshold and initiate swimming. We propose this excitation provides a sensory memory of the stimulus and may be generated by small reverberatory hindbrain networks. Our results uncover fundamental network mechanisms that allow animals to remember brief sensory stimuli and delay simple motor decisions.
Abstract: Many motor responses to sensory input, like locomotion or eye movements, are much slower than reflexes. Can simpler animals provide fundamental answers about the cellular mechanisms for motor decisions? Can we observe the 'accumulation' of excitation to threshold proposed to underlie decision making elsewhere? We explore how somatosensory touch stimulation leads to the decision to swim in hatchling Xenopus tadpoles. Delays measured to swimming in behaving and immobilised tadpoles are long and variable. Activity in their extensively studied sensory and sensory pathway neurons is too short-lived to explain these response delays. Instead, whole-cell recordings from the hindbrain reticulospinal neurons that drive swimming show that these receive prolonged, variable synaptic excitation lasting for nearly a second following a brief stimulus. They fire and initiate swimming when this excitation reaches threshold. Analysis of the summation of excitation requires us to propose extended firing in currently undefined presynaptic hindbrain neurons. Simple models show that a small excitatory recurrent-network inserted in the sensory pathway can mimic this process. We suggest that such a network may generate slow, variable summation of excitation to threshold. This excitation provides a simple memory of the sensory stimulus. It allows temporal and spatial integration of sensory inputs and explains the long, variable delays to swimming. The process resembles the 'accumulation' of excitation proposed for cortical circuits in mammals. We conclude that fundamental elements of sensory memory and decision making are present in the brainstem at a surprisingly early stage in development.
Keywords: Decision-making; Locomotion; Reticulospinal neurons; Somatosensory; Xenopus laevis.
© 2018 The Authors The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures



References
-
- Arber S ( 2012). Motor circuits in action: specification, connectivity, and function. Neuron 74, 975–989. - PubMed
-
- Bogacz R, Wagenmakers EJ, Forstmann BU & Nieuwenhuis S (2010). The neural basis of the speed‐accuracy tradeoff. Trends Neurosci 33, 10–16. - PubMed
-
- Boothby KM & Roberts A (1995). Effects of site and strength of tactile stimulation on the swimming responses of Xenopus laevis embryos. J Zool (Lond) 235, 113–125.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

