A mechanism for bistability in glycosylation
- PMID: 30074989
- PMCID: PMC6093706
- DOI: 10.1371/journal.pcbi.1006348
A mechanism for bistability in glycosylation
Abstract
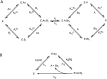
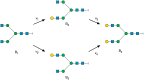
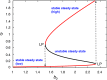
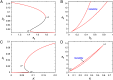
Glycosyltransferases are a class of enzymes that catalyse the posttranslational modification of proteins to produce a large number of glycoconjugate acceptors from a limited number of nucleotide-sugar donors. The products of one glycosyltransferase can be the substrates of several other enzymes, causing a combinatorial explosion in the number of possible glycan products. The kinetic behaviour of systems where multiple acceptor substrates compete for a single enzyme is presented, and the case in which high concentrations of an acceptor substrate are inhibitory as a result of abortive complex formation, is shown to result in non-Michaelian kinetics that can lead to bistability in an open system. A kinetic mechanism is proposed that is consistent with the available experimental evidence and provides a possible explanation for conflicting observations on the β-1,4-galactosyltransferases. Abrupt switching between steady states in networks of glycosyltransferase-catalysed reactions may account for the observed changes in glycosyl-epitopes in cancer cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Garfinkel D, Hess B. Metabolic control mechanisms. VII. A detailed computer model of the glycolytic pathway in ascites cells. J Biol Chem. 1964;239:971–983. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

