A model for rapid, active surveillance for medically-attended acute gastroenteritis within an integrated health care delivery system
- PMID: 30075030
- PMCID: PMC6075775
- DOI: 10.1371/journal.pone.0201805
A model for rapid, active surveillance for medically-attended acute gastroenteritis within an integrated health care delivery system
Abstract
Background: This study presents a novel methodology for estimating all-age, population-based incidence rates of norovirus and other pathogens that contribute to acute gastroenteritis in the United States using an integrated healthcare delivery system as a surveillance platform.
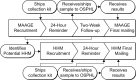
Methods: All cases of medically attended acute gastroenteritis within the delivery system were identified from April 1, 2014 through September 30, 2016. A sample of these eligible patients were selected to participate in two phone-based surveys and to self-collect a stool sample for laboratory testing. To ascertain household transmission patterns, information on household members with acute gastroenteritis was gathered from participants, and symptomatic household members were contacted to participate in a survey and provide stool sample as well.
Results: 54% of individuals who met enrollment criteria agreed to participate, and 76% of those individuals returned a stool sample. Among household members, 85% of eligible individuals agreed to participate, and 68% of those returned a stool sample. Participant demographics were similar to those of the eligible population, although minority racial/ethnic groups were somewhat underrepresented in the final sample.
Conclusions: This study demonstrates the feasibility of conducting acute infectious disease research within an integrated health care delivery system. The surveillance, sampling, recruitment, and data collection methods described here are broadly applicable to conduct baseline and epidemiological assessments, as well as for other research requiring representative samples of stool specimens.
Conflict of interest statement
Funding was received by Takeda Vaccines, Inc. Takeda had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This commercial funding does not alter adherence to all PLOS ONE policies on sharing data and materials.
Figures

• Screw-top plastic container (for the specimen). Collection container will have a label for Health Record Number (HRN), Name, date of birth (DOB), date of collection, time of collection. Recruitment staff will include member’s HRN, Name and DOB before sending kit to participant.
• Card board-and-tissue-paper liner (fits on a toilet seat) with paper bowl (can be added to liner if needed)
• Spoon (to scoop)
• Gloves
• Gauze pad (for use with diapers)
• Alcohol cleaning pad
• Plastic specimen bag (may say "Biohazard") with absorbent towel
• Paper sack
• Cold pack(s)
• Chill Checker button (count of 500 for randomly selected samples)
• Information sheet for keeping specimen at required temperature
• Sending/Returning shipping packet and information sheet
• Shipping label for return sample
• Shipping box



References
-
- Grytdal SP, DeBess E, Lee LE, Blythe D, Ryan P, Biggs C, et al. Incidence of Norovirus and other viral pathogens that cause acute gastroenteritis (AGE) among Kaiser Permanente member populations in the United States, 2012–2013. PLoS One. 2016;11(4):e0148395 10.1371/journal.pone.0148395 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

