Lessons learned from the study of human inborn errors of innate immunity
- PMID: 30075154
- PMCID: PMC6358521
- DOI: 10.1016/j.jaci.2018.07.013
Lessons learned from the study of human inborn errors of innate immunity
Abstract
Innate immunity contributes to host defense through all cell types and relies on their shared germline genetic background, whereas adaptive immunity operates through only 3 main cell types, αβ T cells, γδ T cells, and B cells, and relies on their somatic genetic diversification of antigen-specific responses. Human inborn errors of innate immunity often underlie infectious diseases. The range and nature of infections depend on the mutated gene, the deleteriousness of the mutation, and other ill-defined factors. Most known inborn errors of innate immunity to infection disrupt the development or function of leukocytes other than T and B cells, but a growing number of inborn errors affect cells other than circulating and tissue leukocytes. Here we review inborn errors of innate immunity that have been recently discovered or clarified. We highlight the immunologic implications of these errors.
Keywords: Infection; Toll-like receptors; immunodeficiency; inborn error of immunity; innate immunity; interferon; nuclear factor κ light-chain enhancer of activated B cells; phagocytes; signaling pathway.
Copyright © 2018 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The Authors do not have a Conflict of Interest.
Figures
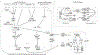

References
-
- Ronald P, Beutler B. Plant and animal sensors of conserved microbial signatures. Science. 2010;330(6007):1061–4. - PubMed
-
- Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol 2006;117(5):979–87. - PubMed
-
- O’Neill L, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13(6):453–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

