Novel antibodies reveal presynaptic localization of C9orf72 protein and reduced protein levels in C9orf72 mutation carriers
- PMID: 30075745
- PMCID: PMC6091050
- DOI: 10.1186/s40478-018-0579-0
Novel antibodies reveal presynaptic localization of C9orf72 protein and reduced protein levels in C9orf72 mutation carriers
Abstract
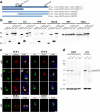
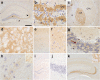
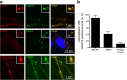
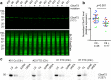
Hexanucleotide repeat expansion in C9orf72 is the most common genetic cause of frontotemporal dementia and amyotrophic lateral sclerosis, but the pathogenic mechanism of this mutation remains unresolved. Haploinsufficiency has been proposed as one potential mechanism. However, insights if and how reduced C9orf72 proteins levels might contribute to disease pathogenesis are still limited because C9orf72 expression, localization and functions in the central nervous system (CNS) are uncertain, in part due to the poor specificity of currently available C9orf72 antibodies.Here, we generated and characterized novel knock-out validated monoclonal rat and mouse antibodies against C9orf72. We found that C9orf72 is a low abundant, cytoplasmic, highly soluble protein with the long 481 amino acid isoform being the predominant, if not exclusively, expressed protein isoform in mouse tissues and human brain. As consequence of the C9orf72 repeat expansion, C9orf72 protein levels in the cerebellum were reduced to 80% in our series of C9orf72 mutation carriers (n = 17) compared to controls (n = 26). However, no associations between cerebellar protein levels and clinical phenotypes were seen. Finally, by utilizing complementary immunohistochemical and biochemical approaches including analysis of human iPSC derived motor neurons, we identified C9orf72, in addition to its association to lysosomes, to be localized to the presynapses and able to interact with all members of the RAB3 protein family, suggestive of a role for C9orf72 in regulating synaptic vesicle functions by potentially acting as guanine nucleotide exchange factor for RAB3 proteins.In conclusion, our findings provide further evidence for haploinsufficiency as potential mechanism in C9orf72 pathogenesis by demonstrating reduced protein levels in C9orf72 mutation carriers and important novel insights into the physiological role of C9orf72 in the CNS. Moreover, the described novel monoclonal C9orf72 antibodies will be useful tools to further dissect the cellular and molecular functions of C9orf72.
Keywords: Amyotrophic lateral sclerosis; C9orf72; Frontotemporal dementia; Frontotemporal lobar degeneration; RAB3; Synaptic vesicles.
Conflict of interest statement
The authors declare that they have no competing interests
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

