2-arachidonyl glycerol modulates astrocytic glutamine synthetase via p38 and ERK1/2 pathways
- PMID: 30075820
- PMCID: PMC6091076
- DOI: 10.1186/s12974-018-1254-x
2-arachidonyl glycerol modulates astrocytic glutamine synthetase via p38 and ERK1/2 pathways
Abstract
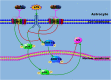
Background: The glutamine synthetase (GS), an astrocyte-specific enzyme, is involved in lipopolysaccharide (LPS)-induced inflammation which activates the mitogen-activated protein kinase (MAPK) signaling. Endocannabinoid 2-arachidonyl glycerol (2-AG) has been described to serve as an endogenous mediator of analgesia and neuroprotection. However, whether 2-AG can directly influence astrocytic GS and MAPK expressions remains unknown.
Methods: In the present study, the effects of 2-AG on astrocytic GS expression, p38 and ERK1/2 expression, cell viability, and apoptosis following LPS exposure were investigated.
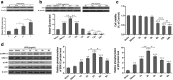
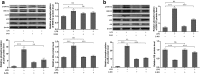
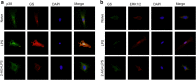
Results: The results revealed that LPS exposure increased GS expression with p38 activation in the early phase and decreased GS expression with activation of ERK1/2, decrease of cell viability, and increase of apoptosis in the late phase. Inhibition of p38 reversed GS increase in the early phase while inhibition of ERK1/2 reversed GS decrease in the late phase induced by LPS exposure. 2-AG protected astrocytes from increase of apoptosis and decrease of cell viability induced by the late phase of LPS exposure. In the early phase of LPS exposure, 2-AG could suppress the increase of GS expression and activation of p38 signaling. In the late phase of LPS exposure, 2-AG could reverse the decrease of GS expression and activation of ERK1/2 induced by LPS.
Conclusion: These findings suggest that 2-AG could maintain the GS expression in astrocytes to a relatively stable level through modulating MAPK signaling and protect astrocytes from LPS exposure.
Keywords: 2-AG; Astrocyte; ERK; Glutamine synthetase; MAPK; p38.
Conflict of interest statement
All efforts were performed to minimize the number of neonatal rats used and their suffering. The procedures were approved by the Animal Care and the Ethic Committee of Animal Usage of Lanzhou University Second Hospital.
Not applicable.
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

