Estrogen Regulation of GRK2 Inactivates Kappa Opioid Receptor Signaling Mediating Analgesia, But Not Aversion
- PMID: 30076211
- PMCID: PMC6136151
- DOI: 10.1523/JNEUROSCI.0653-18.2018
Estrogen Regulation of GRK2 Inactivates Kappa Opioid Receptor Signaling Mediating Analgesia, But Not Aversion
Abstract
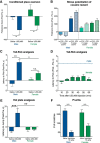
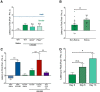
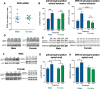
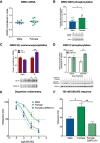
Activation of κ opioid receptors (KORs) produces analgesia and aversion via distinct intracellular signaling pathways, but whether G protein-biased KOR agonists can be designed to have clinical utility will depend on a better understanding of the signaling mechanisms involved. We found that KOR activation produced conditioned place aversion and potentiated CPP for cocaine in male and female C57BL/6N mice. Consistent with this, males and females both showed arrestin-mediated increases in phospho-p38 MAPK following KOR activation. Unlike in males, however, KOR activation had inconsistent analgesic effects in females and KOR increased Gβγ-mediated ERK phosphorylation in males, but not females. KOR desensitization was not responsible for the lack of response in females because neither Grk3 nor Pdyn gene knock-out enhanced analgesia. Instead, responsiveness was estrous cycle dependent because KOR analgesia was evident during low estrogen phases of the cycle and in ovariectomized (OVX) females. Estradiol treatment of OVX females suppressed KOR-mediated analgesia, demonstrating that estradiol was sufficient to blunt Gβγ-mediated KOR signals. G protein-coupled receptor kinase 2 (GRK2) is known to regulate ERK activation, and we found that the inhibitory, phosphorylated form of GRK2 was significantly higher in intact females. GRK2/3 inhibition by CMPD101 increased KOR stimulation of phospho-ERK in females, decreased sex differences in KOR-mediated inhibition of dopamine release, and enhanced mu opioid receptor and KOR-mediated analgesia in females. In OVX females, estradiol increased the association between GRK2 and Gβγ. These studies suggest that estradiol, through increased phosphorylation of GRK2 and possible sequestration of Gβγ by GRK2, blunts G protein-mediated signals.SIGNIFICANCE STATEMENT Chronic pain disorders are more prevalent in females than males, but opioid receptor agonists show inconsistent analgesic efficacy in females. κ opioid receptor (KOR) agonists have been tested in clinical trials for treating pain disorders based on their analgesic properties and low addictive potential. However, the molecular mechanisms underlying sex differences in KOR actions were previously unknown. Our studies identify an intracellular mechanism involving estradiol regulation of G protein-coupled receptor kinase 2 that is responsible for sexually dimorphic analgesic responses following opioid receptor activation. Understanding this mechanism will be critical for developing effective nonaddictive opioid analgesics for use in women and characterizing sexually dimorphic effects in other inhibitory G protein-coupled receptor signaling responses.
Keywords: analgesia; biased signal transduction; estrogen; kappa opioid receptor; morphine; sex differences.
Copyright © 2018 the authors 0270-6474/18/388031-13$15.00/0.
Figures
References
-
- Berkley KJ. (1997) Sex differences in pain. Behav Brain Sci 20:371–380; discussion 435–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
