Cytomegalovirus promotes intestinal macrophage-mediated mucosal inflammation through induction of Smad7
- PMID: 30076393
- PMCID: PMC7405939
- DOI: 10.1038/s41385-018-0041-4
Cytomegalovirus promotes intestinal macrophage-mediated mucosal inflammation through induction of Smad7
Abstract
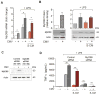
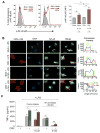
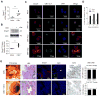
Intestinal macrophages in healthy human mucosa are profoundly down-regulated for inflammatory responses (inflammation anergy) due to stromal TGF-β inactivation of NF-κB. Paradoxically, in cytomegalovirus (CMV) intestinal inflammatory disease, one of the most common manifestations of opportunistic CMV infection, intestinal macrophages mediate severe mucosal inflammation. Here we investigated the mechanism whereby CMV infection promotes macrophage-mediated mucosal inflammation. CMV infected primary intestinal macrophages but did not replicate in the cells or reverse established inflammation anergy. However, CMV infection of precursor blood monocytes, the source of human intestinal macrophages in adults, prevented stromal TGF-β-induced differentiation of monocytes into inflammation anergic macrophages. Mechanistically, CMV up-regulated monocyte expression of the TGF-β antagonist Smad7, blocking the ability of stromal TGF-β to inactivate NF-κB, thereby enabling MyD88 and NF-κB-dependent cytokine production. Smad7 expression also was markedly elevated in mucosal tissue from subjects with CMV colitis and declined after antiviral ganciclovir therapy. Confirming these findings, transfection of Smad7 antisense oligonucleotide into CMV-infected monocytes restored monocyte susceptibility to stromal TGF-β-induced inflammation anergy. Thus, CMV-infected monocytes that recruit to the mucosa, not resident macrophages, are the source of inflammatory macrophages in CMV mucosal disease and implicate Smad7 as a key regulator of, and potential therapeutic target for, CMV mucosal disease.
Conflict of interest statement
Conflict of Interest Statement: The authors declare no conflict of interest.
Figures


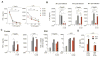
References
-
- Smith PD, Janoff EN. Complications of AIDS and other immunodeficiency states. In: Podolsky DK, Camilleri M, Fitz JG, Kalloo AN, Shanahan F, Wang TC, editors. Yamada’s Textbook of Gastroenterology. 2015. pp. 2588–2611.
-
- Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006;101:2857–2865. - PubMed
-
- Maher MM, Nassar MI. Acute cytomegalovirus infection is a risk factor in refractory and complicated inflammatory bowel disease. Dig Dis Sci. 2009;54:2456–2462. - PubMed
-
- Einhorn L, Ost A. Cytomegalovirus infection of human blood cells. J Infect Dis. 1984;149:207–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

