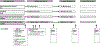Protocol for the Imagine HEALTH Study: Guided imagery lifestyle intervention to improve obesity-related behaviors and salivary cortisol patterns in predominantly Latino adolescents
- PMID: 30076988
- PMCID: PMC8746570
- DOI: 10.1016/j.cct.2018.07.009
Protocol for the Imagine HEALTH Study: Guided imagery lifestyle intervention to improve obesity-related behaviors and salivary cortisol patterns in predominantly Latino adolescents
Abstract
Innovative lifestyle interventions are needed to reduce type 2 diabetes risk in adolescents. This report describes the protocol of the Imagine HEALTH cluster randomized control trial, that tests an intervention based in Self-Determination Theory (SDT) and uses lifestyle education combined with the mind-body, complementary health modality of guided imagery (GI), to address obesity prevention and treatment in predominantly Latino adolescents. The primary aim is to determine the unique effects of each of the three major components of the 12-week lifestyle intervention (lifestyle education, stress reduction guided imagery, and lifestyle behavior guided imagery) compared to control on primary outcomes of physical activity (accelerometry), dietary intake (3-day recall), and stress biomarker levels (salivary cortisol). Secondary aims assess changes compared to controls in psychosocial outcomes (stress, well-being, depression), diabetes-related metabolic outcomes (adiposity, insulin resistance), maintenance of outcome changes for one year post-intervention, and SDT-based mediation of intervention effects. The development and rationale for each of the intervention components, study design, and outcome measurement processes are described. Adolescent participants recruited from four urban schools are cluster randomized by school into one of four arms of the 12-week (3-month) intervention, followed by 6 months of maintenance and 6 months of no contact. Outcome measures are assessed at the end of each period (3-, 9-, and 15-months). Results to date show successful recruitment of 97% of the target study population. Future results will demonstrate the effects of this integrative intervention on primary and secondary outcome measures in adolescents at risk for lifestyle-related metabolic disease.
Trial registration: ClinicalTrials.gov NCT02088294.
Keywords: Adolescent obesity; Council; Guided imagery; Lifestyle behaviors; Lifestyle intervention; Salivary cortisol; Stress.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
References
-
- Goran MI, et al., Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab, 2004. 89(1): p. 207–12. - PubMed
-
- Weigensberg MJ, et al., Decreased beta-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care, 2005. 28(10): p. 2519–24. - PubMed
-
- Cruz ML, et al., The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab, 2004. 89(1): p. 108–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical