Homeostatic Control of Spontaneous Activity in the Developing Auditory System
- PMID: 30077356
- PMCID: PMC6100752
- DOI: 10.1016/j.neuron.2018.07.004
Homeostatic Control of Spontaneous Activity in the Developing Auditory System
Abstract
Neurons in the developing auditory system exhibit spontaneous bursts of activity before hearing onset. How this intrinsically generated activity influences development remains uncertain, because few mechanistic studies have been performed in vivo. We show using macroscopic calcium imaging in unanesthetized mice that neurons responsible for processing similar frequencies of sound exhibit highly synchronized activity throughout the auditory system during this critical phase of development. Spontaneous activity normally requires synaptic excitation of spiral ganglion neurons (SGNs). Unexpectedly, tonotopic spontaneous activity was preserved in a mouse model of deafness in which glutamate release from hair cells is abolished. SGNs in these mice exhibited enhanced excitability, enabling direct neuronal excitation by supporting cell-induced potassium transients. These results indicate that homeostatic mechanisms maintain spontaneous activity in the pre-hearing period, with significant implications for both circuit development and therapeutic approaches aimed at treating congenital forms of deafness arising through mutations in key sensory transduction components.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing financial interests.
Figures



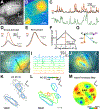

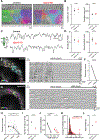
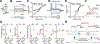
Comment in
-
And the Band Keeps Marching On.Neuron. 2018 Aug 8;99(3):427-429. doi: 10.1016/j.neuron.2018.07.043. Neuron. 2018. PMID: 30092211
-
Auditory advances.Nat Rev Neurosci. 2018 Oct;19(10):579. doi: 10.1038/s41583-018-0052-x. Nat Rev Neurosci. 2018. PMID: 30111776 No abstract available.
References
-
- Bobbin RP (1979). Glutamate and aspartate mimic the afferent transmitter in the cochlea. Exp. Brain Res 34, 389–393. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

