Self-Renewing Trophoblast Organoids Recapitulate the Developmental Program of the Early Human Placenta
- PMID: 30078556
- PMCID: PMC6092984
- DOI: 10.1016/j.stemcr.2018.07.004
Self-Renewing Trophoblast Organoids Recapitulate the Developmental Program of the Early Human Placenta
Abstract
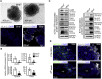
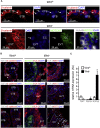
Defective placentation is the underlying cause of various pregnancy complications, such as severe intrauterine growth restriction and preeclampsia. However, studies on human placental development are hampered by the lack of a self-renewing in vitro model that would recapitulate formation of trophoblast progenitors and differentiated subtypes, syncytiotrophoblast (STB) and invasive extravillous trophoblast (EVT), in a 3D orientation. Hence, we established long-term expanding organoid cultures from purified first-trimester cytotrophoblasts (CTBs). Molecular analyses revealed that the CTB organoid cultures (CTB-ORGs) express markers of trophoblast stemness and proliferation and are highly similar to primary CTBs at the level of global gene expression. Whereas CTB-ORGs spontaneously generated STBs, withdrawal of factors for self-renewal induced trophoblast outgrowth, expressing the EVT progenitor marker NOTCH1, and provoked formation of adjacent, distally located HLA-G+ EVTs. In summary, we established human CTB-ORGs that grow and differentiate under defined culture conditions, allowing future human placental disease modeling.
Keywords: Wnt signalling; cell fusion; cytotrophoblast organoids; differentiation; extravillous trophoblast lineage; human placenta; self-renewal.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Aplin J.D. Developmental cell biology of human villous trophoblast: current research problems. Int. J. Dev. Biol. 2010;54:323–329. - PubMed
-
- Apps R., Murphy S.P., Fernando R., Gardner L., Ahad T., Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. - PMC - PubMed
-
- Baczyk D., Dunk C., Huppertz B., Maxwell C., Reister F., Giannoulias D., Kingdom J.C. Bi-potential behaviour of cytotrophoblasts in first trimester chorionic villi. Placenta. 2006;27:367–374. - PubMed
-
- Biadasiewicz K., Sonderegger S., Haslinger P., Haider S., Saleh L., Fiala C., Pollheimer J., Knöfler M. Transcription factor AP-2alpha promotes EGF-dependent invasion of human trophoblast. Endocrinology. 2011;152:1458–1469. - PubMed
-
- Bilban M., Tauber S., Haslinger P., Pollheimer J., Saleh L., Pehamberger H., Wagner O., Knöfler M. Trophoblast invasion: assessment of cellular models using gene expression signatures. Placenta. 2010;31:989–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

