Apicomplexa Cell Cycles: Something Old, Borrowed, Lost, and New
- PMID: 30078701
- PMCID: PMC6157590
- DOI: 10.1016/j.pt.2018.07.006
Apicomplexa Cell Cycles: Something Old, Borrowed, Lost, and New
Erratum in
-
Apicomplexa Cell Cycles: Something Old, Borrowed, Lost, and New: (Trends in Parasitology 34, 759-771; 2018).Trends Parasitol. 2018 Nov;34(11):1012-1013. doi: 10.1016/j.pt.2018.09.002. Epub 2018 Oct 9. Trends Parasitol. 2018. PMID: 30314807 No abstract available.
Abstract
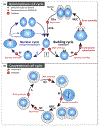
Increased parasite burden is linked to the severity of clinical disease caused by Apicomplexa parasites such as Toxoplasma gondii, Plasmodium spp, and Cryptosporidium. Pathogenesis of apicomplexan infections is greatly affected by the growth rate of the parasite asexual stages. This review discusses recent advances in deciphering the mitotic structures and cell cycle regulatory factors required by Apicomplexa parasites to replicate. As the molecular details become clearer, it is evident that the highly unconventional cell cycles of these parasites is a blending of many ancient and borrowed elements, which were then adapted to enable apicomplexan proliferation in a wide variety of different animal hosts.
Keywords: Apicomplexa; Toxoplasma gondii; cell cycle; cyclin-dependent kinase; evolution; mitosis.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
The Birth of Red Complex Plastids: One, Three, or Four Times?Trends Parasitol. 2018 Nov;34(11):923-925. doi: 10.1016/j.pt.2018.09.001. Epub 2018 Sep 17. Trends Parasitol. 2018. PMID: 30237040 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

