Heteromeric RNP Assembly at LINEs Controls Lineage-Specific RNA Processing
- PMID: 30078707
- PMCID: PMC6108849
- DOI: 10.1016/j.cell.2018.07.001
Heteromeric RNP Assembly at LINEs Controls Lineage-Specific RNA Processing
Abstract
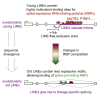
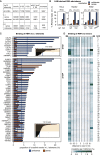
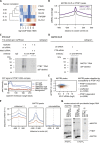
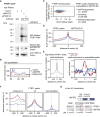
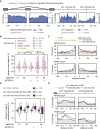
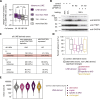
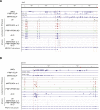
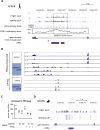
Long mammalian introns make it challenging for the RNA processing machinery to identify exons accurately. We find that LINE-derived sequences (LINEs) contribute to this selection by recruiting dozens of RNA-binding proteins (RBPs) to introns. This includes MATR3, which promotes binding of PTBP1 to multivalent binding sites within LINEs. Both RBPs repress splicing and 3' end processing within and around LINEs. Notably, repressive RBPs preferentially bind to evolutionarily young LINEs, which are located far from exons. These RBPs insulate the LINEs and the surrounding intronic regions from RNA processing. Upon evolutionary divergence, changes in RNA motifs within LINEs lead to gradual loss of their insulation. Hence, older LINEs are located closer to exons, are a common source of tissue-specific exons, and increasingly bind to RBPs that enhance RNA processing. Thus, LINEs are hubs for the assembly of repressive RBPs and also contribute to the evolution of new, lineage-specific transcripts in mammals. VIDEO ABSTRACT.
Keywords: CLIP; LINE repeats; MATR3; PTBP1; alternative polyadenylation; cryptic exons; evolution; exonogenesis; multivalency; splicing.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Bakkar N., Kovalik T., Lorenzini I., Spangler S., Lacoste A., Sponaugle K., Ferrante P., Argentinis E., Sattler R., Bowser R. Artificial intelligence in neurodegenerative disease research: use of IBM Watson to identify additional RNA-binding proteins altered in amyotrophic lateral sclerosis. Acta Neuropathol. 2018;135:227–247. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

