Sensory Neuron Diversity in the Inner Ear Is Shaped by Activity
- PMID: 30078709
- PMCID: PMC6150604
- DOI: 10.1016/j.cell.2018.07.007
Sensory Neuron Diversity in the Inner Ear Is Shaped by Activity
Abstract
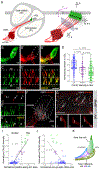
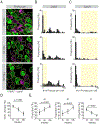
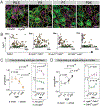
In the auditory system, type I spiral ganglion neurons (SGNs) convey complex acoustic information from inner hair cells (IHCs) to the brainstem. Although SGNs exhibit variation in physiological and anatomical properties, it is unclear which features are endogenous and which reflect input from synaptic partners. Using single-cell RNA sequencing, we derived a molecular classification of mouse type I SGNs comprising three subtypes that express unique combinations of Ca2+ binding proteins, ion channel regulators, guidance molecules, and transcription factors. Based on connectivity and susceptibility to age-related loss, these subtypes correspond to those defined physiologically. Additional intrinsic differences among subtypes and across the tonotopic axis highlight an unexpectedly active role for SGNs in auditory processing. SGN identities emerge postnatally and are disrupted in a mouse model of deafness that lacks IHC-driven activity. These results elucidate the range, nature, and origins of SGN diversity, with implications for treatment of congenital deafness.
Keywords: Vglut3; activity-dependent development; auditory; neuron heterogeneity; neuronal subtypes; single-cell RNA-seq; spiral ganglion neurons; spontaneous activity.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures




Comment in
-
Auditory advances.Nat Rev Neurosci. 2018 Oct;19(10):579. doi: 10.1038/s41583-018-0052-x. Nat Rev Neurosci. 2018. PMID: 30111776 No abstract available.
References
-
- Adamson CL, Reid M. a., Mo ZL, Bowne-English J, and Davis RL (2002). Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J. Comp. Neurol 447, 331–350. - PubMed
-
- El Barbary A (1991). Auditory nerve of the normal and jaundiced rat. I. Spontaneous discharge rate and cochlear nerve histology. Hear. Res 54, 75–90. - PubMed
-
- Berglund AM, and Ryugo DK (1987). Hair cell innervation by spiral ganglion neurons in the mouse. J. Comp. Neurol 255, 560–570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

