Metabolomic Analysis of Mouse Brain after a Transient Middle Cerebral Artery Occlusion by Mass Spectrometry Imaging
- PMID: 30078821
- PMCID: PMC6156127
- DOI: 10.2176/nmc.oa.2018-0054
Metabolomic Analysis of Mouse Brain after a Transient Middle Cerebral Artery Occlusion by Mass Spectrometry Imaging
Abstract
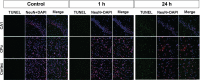
We performed metabolomic analyses of mouse brain using a transient middle cerebral artery occlusion (tMCAO) model with Matrix Assisted Laser Desorption/Ionization (MALDI)-mass spectrometry imaging (MSI) to reveal metabolite changes after cerebral ischemia. We selected and analyzed three metabolites, namely creatine (Cr), phosphocreatine (P-Cr), and ceramides (Cer), because these metabolites contribute to cell life and death. Eight-week-old male C57BL/6J mice were subjected to tMCAO via the intraluminal blockade of the middle cerebral artery (MCA) and reperfusion 60 min after the induction of ischemia. Each mouse was randomly assigned to one of the three groups; the groups were defined by the survival period after reperfusion: control, 1 h, and 24 h. Corrected samples were analyzed using MALDI-MSI. Results of MSI analysis showed the presence of several ionized substances and revealed spatial changes in some metabolites identified as precise substances, including Cr, P-Cr, Cer d18:1/18:0, phosphatidylcholine, L-glutamine, and L-histidine. Cr, P-Cr, and Cer d18:1/18:0 were changed after tMCAO, and P-Cr and Cer d18:1/18:0 accumulated over time in ischemic cores and surrounding areas following ischemia onset. The upregulation of P-Cr and Cer d18:1/18:0 was detected 1 h after tMCAO when no changes were evident on hematoxylin and eosin staining and immunofluorescence assay. P-Cr and Cer d18:1/18:0 can serve as neuroprotective therapies because they are biomarker candidates for cerebral ischemia.
Keywords: ceramide; cerebral ischemia; mass spectrometry imaging; metabolomic analysis; phosphocreatine.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures



References
-
- Mulder IA, Esteve C, Wermer MJ, et al. : Funnel-freezing versus heat-stabilization for the visualization of metabolites by mass spectrometry imaging in a mouse stroke model. Proteomics 16: 1652–1659, 2016 - PubMed
-
- Pohjanen E, Thysell E, Jonsson P, et al. : A multivariate screening strategy for investigating metabolic effects of strenuous physical exercise in human serum. J Proteome Res 6: 2113–2120, 2007 - PubMed
-
- Werner E, Croixmarie V, Umbdenstock T, et al. : Mass spectrometry-based metabolomics: accelerating the characterization of discriminating signals by combining statistical correlations and ultrahigh resolution. Anal Chem 80: 4918–4932, 2008 - PubMed
-
- Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM: Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med 7: 493–496, 2001 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

