MicroRNAs and immunity in periodontal health and disease
- PMID: 30078842
- PMCID: PMC6080405
- DOI: 10.1038/s41368-018-0025-y
MicroRNAs and immunity in periodontal health and disease
Abstract
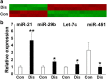
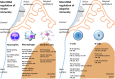
MicroRNAs (miRNAs) are critical regulators of the host immune and inflammatory response against bacterial pathogens. In the present review, we discuss target genes, target gene functions, the potential regulatory role of miRNAs in periodontal tissues, and the potential role of miRNAs as biomarkers and therapeutics. In periodontal disease, miRNAs exert control over all aspects of innate and adaptive immunity, including the functions of neutrophils, macrophages, dendritic cells and T and B cells. Previous human studies have highlighted some key miRNAs that are dysregulated in periodontitis patients. In the present study, we mapped the major miRNAs that were altered in our reproducible periodontitis mouse model relative to control animals. The miRNAs that were upregulated as a result of periodontal disease in both human and mouse studies included miR-15a, miR-29b, miR-125a, miR-146a, miR-148/148a and miR-223, whereas miR-92 was downregulated. The association of individual miRNAs with unique aspects of periodontal disease and their stability in gingival crevicular fluid underscores their potential as markers for periodontal disease progression or healthy restitution. Moreover, miRNA therapeutics hold great promise for the future of periodontal therapy because of their ability to modulate the immune response to infection when applied in conjunction with synthetic antagomirs and/or relatively straightforward delivery strategies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

