Modulation of Phytohormone Signaling: A Primary Function of Flavonoids in Plant-Environment Interactions
- PMID: 30079075
- PMCID: PMC6062965
- DOI: 10.3389/fpls.2018.01042
Modulation of Phytohormone Signaling: A Primary Function of Flavonoids in Plant-Environment Interactions
Abstract
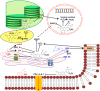
The old observation that plants preferentially synthesize flavonoids with respect to the wide range of phenylpropanoid structures when exposed to high doses of UV-B radiation has supported the view that flavonoids are primarily involved in absorbing the shortest solar wavelengths in photoprotection. However, there is compelling evidence that the biosynthesis of flavonoids is similarly upregulated in response to high photosynthetically active radiation in the presence or in the absence of UV-radiation, as well as in response to excess metal ions and photosynthetic redox unbalance. This supports the hypothesis that flavonoids may play prominent roles as scavengers of reactive oxygen species (ROS) generated by light excess. These 'antioxidant' functions of flavonoids appears robust, as maintained between different life kingdoms, e.g., plants and animals. The ability of flavonoids to buffer stress-induced large alterations in ROS homeostasis and, hence, to modulate the ROS-signaling cascade, is at the base of well-known functions of flavonoids as developmental regulators in both plants and animals. There is both long and very recent evidence indeed that, in plants, flavonoids may strongly affect phytohormone signaling, e.g., auxin and abscisic acid signaling. This function is served by flavonoids in a very low (nM) concentration range and involves the ability of flavonoids to inhibit the activity of a wide range of protein kinases, including but not limited to mitogen-activated protein kinases, that operate downstream of ROS in the regulation of cell growth and differentiation. For example, flavonoids inhibit the transport of auxin acting on serine-threonine PINOID (PID) kinases that regulate the localization of auxin efflux facilitators PIN-formed (PIN) proteins. Flavonoids may also determine auxin gradients at cellular and tissue levels, and the consequential developmental processes, by reducing auxin catabolism. Recent observations lead to the hypothesis that regulation/modulation of auxin transport/signaling is likely an ancestral function of flavonoids. The antagonistic functions of flavonoids on ABA-induced stomatal closure also offer novel hypotheses on the functional role of flavonoids in plant-environment interactions, in early as well as in modern terrestrial plants. Here, we surmise that the regulation of phytohormone signaling might have represented a primary function served by flavonols for the conquest of land by plants and it is still of major significance for the successful acclimation of modern terrestrial plants to a severe excess of radiant energy.
Keywords: abscisic acid (ABA); auxin; early land plants; flavonols; mitogen-activated protein kinases (MAPKs); reactive oxygen species (ROS).
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

