Selective activation of organocatalysts by specific signals
- PMID: 30079215
- PMCID: PMC6050528
- DOI: 10.1039/c8sc02019a
Selective activation of organocatalysts by specific signals
Abstract
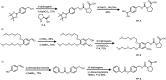
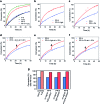
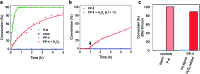
Reminiscent of signal transduction in biological systems, artificial catalysts whose activity can be controlled by physical or chemical signals would be of high interest in the design of chemical systems that can respond to their environment. Self-immolative chemistry offers a generic method for the development of catalysts that can be activated by different signals. To demonstrate the versatility of that concept, we synthesized organocatalysts that can be activated by three different signals and that can be used to control two different reactions. In this way the organocatalyst proline is designed as a pro-catalyst that is activated either by the chemical signal H2O2, by light or by the enzyme penicillin acylase. The pro-catalysts were used to exert temporal control over the rate of an aldol reaction and a Michael reaction.
Figures

References
-
- Whitehead E. Prog. Biophys. Mol. Biol. 1970;21:321. - PubMed
-
- Traut T., Allosteric regulatory enzymes, Springer, Boston, 2008.
-
- Blanco V., Leigh D. A., Marcos V. Chem. Soc. Rev. 2015;44:5341. - PubMed
-
- Stoll R. S., Hecht S. Angew. Chem., Int. Ed. 2010;49:5054. - PubMed
- Neri S., Garcia Martin S., Pezzato C., Prins L. J. J. Am. Chem. Soc. 2017;139:1794. - PubMed
- Zhao H., Sen S., Udayabhaskararao T., Sawczyk M., Kučanda K., Manna D., Kundu P. K., Lee J.-W., Král P., Klajn R. Nat. Nanotechnol. 2016;11:82. - PubMed
- Maity C., Hendriksen W. E., van Esch J. H., Eelkema R. Angew. Chem., Int. Ed. 2015;54:998. - PubMed
-
- Gianneschi N. C., Bertin P. A., Nguyen S. T., Mirkin C. A., Zakharov L. N., Rheingold A. L. J. Am. Chem. Soc. 2003;125:10508. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

