Primary Prophylaxis to Prevent the Development of Hepatic Encephalopathy in Cirrhotic Patients with Acute Variceal Bleeding
- PMID: 30079329
- PMCID: PMC6069577
- DOI: 10.1155/2018/3015891
Primary Prophylaxis to Prevent the Development of Hepatic Encephalopathy in Cirrhotic Patients with Acute Variceal Bleeding
Abstract
Background and aim: Variceal bleeding is the second most important precipitating factor related to the development of episodic hepatic encephalopathy; but to date there are no recommendations to prevent this complication. The aim of this study was to compare if primary prophylaxis with lactulose or L-ornithine L-aspartate or rifaximin, in cirrhotic patients with variceal bleeding, is better than placebo for avoiding the development of hepatic encephalopathy.
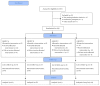
Methods: A randomized, double-blind, placebo-controlled clinical trial (ClinicalTrials.gov identifier: NCT02158182) which included cirrhotic patients with variceal bleeding, without minimal or clinical hepatic encephalopathy at admission.
Findings: 87 patients were randomized to one of four groups. The basal characteristics were similar between groups. Comparatively with placebo, the frequency with regard to the development of hepatic encephalopathy was as follows: lactulose (54.5% versus 27.3%; OR = 0.3, 95% CI 0.09-1.0; P = 0.06); L-ornithine L-aspartate (54.5% versus 22.7%, OR = 0.2, 95% CI 0.06-0.88; P = 0.03); rifaximin (54.5% versus 23.8%; OR = 0.3, 95% CI 0.07-0.9; P = 0.04). There was no significant difference between the three groups receiving any antiammonium drug (P = 0.94). In the group receiving lactulose, 59.1% had diarrhea, and 45.5% had abdominal discomfort, bloating, and flatulence. Two patients (10%) treated with lactulose and a patient (4.5%) in the placebo group developed spontaneous bacterial peritonitis due to E. coli; one of them died due to recurrent variceal bleeding. There were no other adverse effects.
Conclusions: Antiammonium drugs, particularly L-ornithine L-aspartate and rifaximin, proved to be effective in preventing the development of hepatic encephalopathy in those cirrhotic patients with variceal bleeding.
Figures
References
-
- Ferenci P., Lockwood A., Mullen K., Tarter R., Weissenborn K., Blei A. T. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the Working Party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–721. doi: 10.1053/jhep.2002.31250. - DOI - PubMed
-
- Ong J. P., Oehler G., Krüger-Jansen C., Lambert-Baumann J., Younossi Z. M. Oral L-ornithine-L-aspartate improves health-related quality of life in cirrhotic patients with hepatic encephalopathy: An open-label, prospective, multicentre observational study. Clinical Drug Investigation. 2011;31(4):213–220. doi: 10.2165/11586700-000000000-00000. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

