Drosophila CaV2 channels harboring human migraine mutations cause synapse hyperexcitability that can be suppressed by inhibition of a Ca2+ store release pathway
- PMID: 30080864
- PMCID: PMC6095605
- DOI: 10.1371/journal.pgen.1007577
Drosophila CaV2 channels harboring human migraine mutations cause synapse hyperexcitability that can be suppressed by inhibition of a Ca2+ store release pathway
Abstract
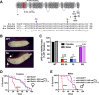
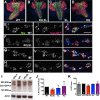
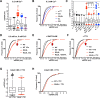
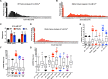
Gain-of-function mutations in the human CaV2.1 gene CACNA1A cause familial hemiplegic migraine type 1 (FHM1). To characterize cellular problems potentially triggered by CaV2.1 gains of function, we engineered mutations encoding FHM1 amino-acid substitutions S218L (SL) and R192Q (RQ) into transgenes of Drosophila melanogaster CaV2/cacophony. We expressed the transgenes pan-neuronally. Phenotypes were mild for RQ-expressing animals. By contrast, single mutant SL- and complex allele RQ,SL-expressing animals showed overt phenotypes, including sharply decreased viability. By electrophysiology, SL- and RQ,SL-expressing neuromuscular junctions (NMJs) exhibited enhanced evoked discharges, supernumerary discharges, and an increase in the amplitudes and frequencies of spontaneous events. Some spontaneous events were gigantic (10-40 mV), multi-quantal events. Gigantic spontaneous events were eliminated by application of TTX-or by lowered or chelated Ca2+-suggesting that gigantic events were elicited by spontaneous nerve firing. A follow-up genetic approach revealed that some neuronal hyperexcitability phenotypes were reversed after knockdown or mutation of Drosophila homologs of phospholipase Cβ (PLCβ), IP3 receptor, or ryanodine receptor (RyR)-all factors known to mediate Ca2+ release from intracellular stores. Pharmacological inhibitors of intracellular Ca2+ store release produced similar effects. Interestingly, however, the decreased viability phenotype was not reversed by genetic impairment of intracellular Ca2+ release factors. On a cellular level, our data suggest inhibition of signaling that triggers intracellular Ca2+ release could counteract hyperexcitability induced by gains of CaV2.1 function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87(3):543–52. - PubMed
-
- Kors EE, Terwindt GM, Vermeulen FL, Fitzsimons RB, Jardine PE, Heywood P, et al. Delayed cerebral edema and fatal coma after minor head trauma: role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann Neurol. 2001;49(6):753–60. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

