Interaction between the cellular E3 ubiquitin ligase SIAH-1 and the viral immediate-early protein ICP0 enables efficient replication of Herpes Simplex Virus type 2 in vivo
- PMID: 30080903
- PMCID: PMC6078308
- DOI: 10.1371/journal.pone.0201880
Interaction between the cellular E3 ubiquitin ligase SIAH-1 and the viral immediate-early protein ICP0 enables efficient replication of Herpes Simplex Virus type 2 in vivo
Abstract
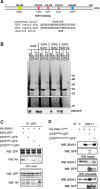
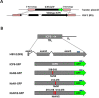
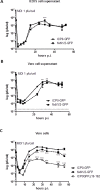
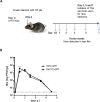
Herpes Simplex Virus type 2 (HSV-2) is a neurotropic human pathogen. Upon de novo infection, the viral infected cell protein 0 (ICP0) is immediately expressed and interacts with various cellular components during the viral replication cycle. ICP0 is a multifunctional regulatory protein that has been shown to be important for both efficient viral replication and virus reactivation from latency. In particular, as previously demonstrated in transfected tissue culture models, ICP0 interacts with the cellular E3 ubiquitin ligase SIAH-1, which targets ICP0 for proteasomal degradation. However, the consequence of this virus-host interaction during the establishment of HSV-2 infection in vivo has not yet been elucidated. Here we confirmed that ICP0 of HSV-2 interacts with SIAH-1 via two conserved PxAxVxP amino acid binding motifs. We also demonstrate in vitro that a SIAH-1 binding-deficient HSV-2 strain, constructed by homologous recombination technology, exhibits an attenuated growth curve and impaired DNA and protein synthesis. This attenuated phenotype was also confirmed in an in vivo ocular infection mouse model. Specifically, viral load of the SIAH-1 binding-deficient HSV-2 mutant was significantly reduced in the trigeminal ganglia and brain stem at day 5 and 7 post infection. Our findings indicate that the interplay between ICP0 and SIAH-1 is important for efficient HSV-2 replication in vivo, thereby affecting viral dissemination kinetics in newly infected organisms, and possibly revealing novel targets for antiviral therapy.
Conflict of interest statement
The commercial affiliation of an author of this study [MME] (Biomedizinische Forschungsgesellschaft mbH, Vienna, Austria) does not alter our adherence to all PLOS ONE policies on sharing data and materials.
Figures



References
-
- Roizman B, Knipe DM (2001) Herpes Simplex viruses and their replication In: Knipe DM, Howley P, Griffin DE, Lamb RA, Martin MA et al., editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; pp. 2399–2459.
-
- Sandri-Goldin RM (2007) Initiation of transcription and RNA synthesis, processing and transport in HSV and VZV infected cells. NBK47363 [bookaccession]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

