Onset and Progression of Human Osteoarthritis-Can Growth Factors, Inflammatory Cytokines, or Differential miRNA Expression Concomitantly Induce Proliferation, ECM Degradation, and Inflammation in Articular Cartilage?
- PMID: 30081513
- PMCID: PMC6121276
- DOI: 10.3390/ijms19082282
Onset and Progression of Human Osteoarthritis-Can Growth Factors, Inflammatory Cytokines, or Differential miRNA Expression Concomitantly Induce Proliferation, ECM Degradation, and Inflammation in Articular Cartilage?
Abstract
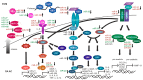
Osteoarthritis (OA) is a degenerative whole joint disease, for which no preventative or therapeutic biological interventions are available. This is likely due to the fact that OA pathogenesis includes several signaling pathways, whose interactions remain unclear, especially at disease onset. Early OA is characterized by three key events: a rarely considered early phase of proliferation of cartilage-resident cells, in contrast to well-established increased synthesis, and degradation of extracellular matrix components and inflammation, associated with OA progression. We focused on the question, which of these key events are regulated by growth factors, inflammatory cytokines, and/or miRNA abundance. Collectively, we elucidated a specific sequence of the OA key events that are described best as a very early phase of proliferation of human articular cartilage (AC) cells and concomitant anabolic/catabolic effects that are accompanied by incipient pro-inflammatory effects. Many of the reviewed factors appeared able to induce one or two key events. Only one factor, fibroblast growth factor 2 (FGF2), is capable of concomitantly inducing all key events. Moreover, AC cell proliferation cannot be induced and, in fact, is suppressed by inflammatory signaling, suggesting that inflammatory signaling cannot be the sole inductor of all early OA key events, especially at disease onset.
Keywords: SMA- and MAD-related protein; articular cartilage; early osteoarthritis; fibroblast growth factor 2; interleukin; miRNA; mitogen activated protein kinase; nuclear factor kappa B; proliferation; transforming growth factor β.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- McAlindon T.E., Bannuru R.R., Sullivan M.C., Arden N.K., Berenbaum F., Bierma-Zeinstra S.M., Hawker G.A., Henrotin Y., Hunter D.J., Kawaguchi H., et al. Oarsi guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. - DOI - PubMed
-
- Ondresik M., Azevedo Maia F.R., da Silva Morais A., Gertrudes A.C., Dias Bacelar A.H., Correia C., Goncalves C., Radhouani H., Amandi Sousa R., Oliveira J.M., et al. Management of knee osteoarthritis. Current status and future trends. Biotechnol. Bioeng. 2017;114:717–739. doi: 10.1002/bit.26182. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

