AIE/ACQ Effects in Two DR/NIR Emitters: A Structural and DFT Comparative Analysis
- PMID: 30081544
- PMCID: PMC6222639
- DOI: 10.3390/molecules23081947
AIE/ACQ Effects in Two DR/NIR Emitters: A Structural and DFT Comparative Analysis
Abstract
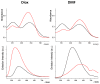
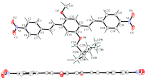
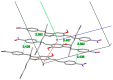
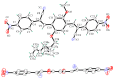
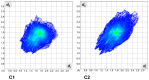
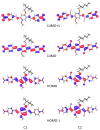
The effects of aggregation-induced emission (AIE) and of aggregation caused quenching (ACQ) were observed and discussed on two solid materials based on a phenylenevinylene (PV) and a dicyano-PV structure. The brightest emitter in solid films shows a high fluorescence quantum yield in the deep red/near IR (DR/NIR) region (75%). The spectroscopic properties of the two crystalline solids have been described and compared in terms of crystallographic data and time dependent DFT analysis. The influence of the cyano-substituents on AIE/ACQ mechanism activation was discussed.
Keywords: AIE/ACQ; DFT; DR/NIR emitter; PLQY.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
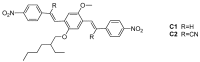




References
-
- Bruno A., Villani F., Grimaldi I., Loffredo F., Morvillo P., Diana R., Haque S., Minarini C. Morphological and spectroscopic characterizations of inkjet-printed poly(3-hexylthiophene-2,5-diyl): Phenyl-C61-butyric acid methyl ester blends for organic solar cell applications. Thin Solid Films. 2014;560:14–19. doi: 10.1016/j.tsf.2013.11.150. - DOI
-
- Ghaemy M., Alizadeh R. Synthesis, characterization and photophysical properties of organosoluble and thermally stable polyamides containing pendent N-carbazole group. React. Funct. Polym. 2011;71:425–432. doi: 10.1016/j.reactfunctpolym.2010.12.015. - DOI
-
- Morvillo P., Ricciardi R., Nenna G., Bobeico E., Diana R., Minarini C. Elucidating the origin of the improved current output in inverted polymer solar cells. Sol. Energy Mater. Sol. Cells. 2016;152:51–58. doi: 10.1016/j.solmat.2016.03.027. - DOI
-
- Rahman D.M., Hameed M.F.O., Obayya S. Light harvesting improvement of polymer solar cell through nanohole photoactive layer. Opt. Quantum Electron. 2015;47:1443–1449. doi: 10.1007/s11082-014-0110-1. - DOI
-
- Bruno A., Borriello C., Di Luccio T., Nenna G., Sessa L., Concilio S., Haque S.A., Minarini C. White light-emitting nanocomposites based on an oxadiazole–carbazole copolymer (POC) and Inp/Zns quantum dots. J. Nanopart. Res. 2013;15 doi: 10.1007/s11051-013-2085-4. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

