Studies on Aminoglycoside Susceptibility Identify a Novel Function of KsgA To Secure Translational Fidelity during Antibiotic Stress
- PMID: 30082289
- PMCID: PMC6153849
- DOI: 10.1128/AAC.00853-18
Studies on Aminoglycoside Susceptibility Identify a Novel Function of KsgA To Secure Translational Fidelity during Antibiotic Stress
Abstract
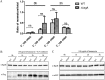
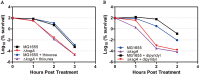
Antibiotic resistance has become a global crisis. Studies on the mechanism of bacterial tolerance to antibiotics will not only increase our conceptual understanding of bacterial death but also provide potential targets for novel inhibitors. We screened a mutant library containing a full set of in-frame deletion mutants of Escherichia coli K-12 and identified 140 genes that possibly contribute to gentamicin tolerance. The deletion of ksgA increased the inhibition and killing potency against mid-log-phase bacteria by aminoglycosides. Initially identified as a 16S rRNA methyltransferase, KsgA also has additional functions as a ribosomal biogenesis factor and a DNA glycosylase. We found that the methyltransferase activity of KsgA is responsible for the tolerance, as demonstrated by a site-directed mutagenesis analysis. In contrast to the mechanism for cold sensitivity, the decreased tolerance to aminoglycoside is not related to the failure of ribosomal biogenesis. Furthermore, the DNA glycosylase activity of KsgA contributes minimally to kanamycin tolerance. Importantly, we discovered that KsgA secures protein translational fidelity upon kanamycin killing, in contrast to its role during cold stress and kasugamycin treatment. The results suggest that the compromise in protein translational fidelity in the absence of KsgA is the root cause of an increased sensitivity to a bactericidal aminoglycoside. In addition, KsgA in the pathogenic Acinetobacter baumannii contributes not only to the tolerance against aminoglycoside killing but also to virulence in the host, warranting its potential application as a target for inhibitors that potentiate aminoglycoside therapeutic killing as well as disarm bacterial virulence simultaneously.
Keywords: Acinetobacter baumannii; KsgA; aminoglycosides; translational fidelity; virulence.
Copyright © 2018 American Society for Microbiology.
Figures





References
-
- O'Neill J. 2014. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. The Review on Antimicrobial Resistance, London, United Kingdom.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

