Genotype-targeted local therapy of glioma
- PMID: 30082399
- PMCID: PMC6130372
- DOI: 10.1073/pnas.1805751115
Genotype-targeted local therapy of glioma
Abstract
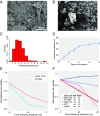
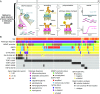
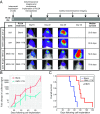
Aggressive neurosurgical resection to achieve sustained local control is essential for prolonging survival in patients with lower-grade glioma. However, progression in many of these patients is characterized by local regrowth. Most lower-grade gliomas harbor isocitrate dehydrogenase 1 (IDH1) or IDH2 mutations, which sensitize to metabolism-altering agents. To improve local control of IDH mutant gliomas while avoiding systemic toxicity associated with metabolic therapies, we developed a precision intraoperative treatment that couples a rapid multiplexed genotyping tool with a sustained release microparticle (MP) drug delivery system containing an IDH-directed nicotinamide phosphoribosyltransferase (NAMPT) inhibitor (GMX-1778). We validated our genetic diagnostic tool on clinically annotated tumor specimens. GMX-1778 MPs showed mutant IDH genotype-specific toxicity in vitro and in vivo, inducing regression of orthotopic IDH mutant glioma murine models. Our strategy enables immediate intraoperative genotyping and local application of a genotype-specific treatment in surgical scenarios where local tumor control is paramount and systemic toxicity is therapeutically limiting.
Keywords: glioma; intraoperative diagnostics; local therapy; metabolic therapeutics.
Conflict of interest statement
Conflict of interest statement: G.M.S., A.R.K., R.L., G.T., and D.P.C. have filed a provisional patent application for this technology.
Figures


Comment in
-
Personalized therapeutic delivery in the neurosurgical operating room.Proc Natl Acad Sci U S A. 2018 Sep 4;115(36):8846-8848. doi: 10.1073/pnas.1812559115. Epub 2018 Aug 20. Proc Natl Acad Sci U S A. 2018. PMID: 30127023 Free PMC article. No abstract available.
References
-
- Balss J, et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. - PubMed
-
- Schwartzentruber J, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. - PubMed
-
- Schindler G, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

