Molecular Structure of the Hair Cell Mechanoelectrical Transduction Complex
- PMID: 30082452
- PMCID: PMC6496331
- DOI: 10.1101/cshperspect.a033167
Molecular Structure of the Hair Cell Mechanoelectrical Transduction Complex
Abstract
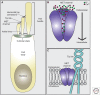
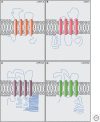
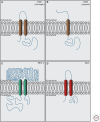
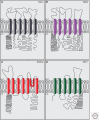
Cochlear hair cells employ mechanically gated ion channels located in stereocilia that open in response to sound wave-induced motion of the basilar membrane, converting mechanical stimulation to graded changes in hair cell membrane potential. Membrane potential changes in hair cells cause neurotransmitter release from hair cells that initiate electrical signals in the nerve terminals of afferent fibers from spiral ganglion neurons. These signals are then propagated within the central nervous system (CNS) to mediate the sensation of hearing. Recent studies show that the mechanoelectrical transduction (MET) machinery of hair cells is formed by an ensemble of proteins. Candidate components forming the MET channel have been identified, but none alone fulfills all criteria necessary to define them as pore-forming subunits of the MET channel. We will review here recent findings on the identification and function of proteins that are components of the MET machinery in hair cells and consider remaining open questions.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
-
- Adato A, Michel V, Kikkawa Y, Reiners J, Alagramam KN, Weil D, Yonekawa H, Wolfrum U, El-Amraoui A, Petit C. 2005. Interactions in the network of Usher syndrome type 1 proteins. Hum Mol Genet 14: 347–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
