Relationship between the Viable but Nonculturable State and Antibiotic Persister Cells
- PMID: 30082460
- PMCID: PMC6153661
- DOI: 10.1128/JB.00249-18
Relationship between the Viable but Nonculturable State and Antibiotic Persister Cells
Abstract
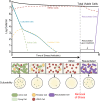
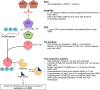
Bacteria have evolved numerous means of survival in adverse environments with dormancy, as represented by "persistence" and the "viable but nonculturable" (VBNC) state, now recognized to be common modes for such survival. VBNC cells have been defined as cells which, induced by some stress, become nonculturable on media that would normally support their growth but which can be demonstrated by various methods to be alive and capable of returning to a metabolically active and culturable state. Persister cells have been described as a population of cells which, while not being antibiotic resistant, are antibiotic tolerant. This drug-tolerant phenotype is thought to be a result of stress-induced and stochastic physiological changes as opposed to mutational events leading to true resistance. In this review, we describe these two dormancy strategies, characterize the molecular underpinnings of each state, and highlight the similarities and differences between them. We believe these survival modes represent a continuum between actively growing and dead cells, with VBNC cells being in a deeper state of dormancy than persister cells.
Keywords: VBNC; antibiotic resistance; antibiotic tolerance; dormancy; drug discovery; persistence; persister; viable but nonculturable.
Copyright © 2018 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

