A Visually Apparent and Quantifiable CT Imaging Feature Identifies Biophysical Subtypes of Pancreatic Ductal Adenocarcinoma
- PMID: 30082477
- PMCID: PMC6279613
- DOI: 10.1158/1078-0432.CCR-17-3668
A Visually Apparent and Quantifiable CT Imaging Feature Identifies Biophysical Subtypes of Pancreatic Ductal Adenocarcinoma
Abstract
Purpose: Pancreatic ductal adenocarcinoma (PDAC) is a heterogeneous disease with variable presentations and natural histories of disease. We hypothesized that different morphologic characteristics of PDAC tumors on diagnostic computed tomography (CT) scans would reflect their underlying biology.
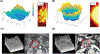
Experimental design: We developed a quantitative method to categorize the PDAC morphology on pretherapy CT scans from multiple datasets of patients with resectable and metastatic disease and correlated these patterns with clinical/pathologic measurements. We modeled macroscopic lesion growth computationally to test the effects of stroma on morphologic patterns, hypothesizing that the balance of proliferation and local migration rates of the cancer cells would determine tumor morphology.
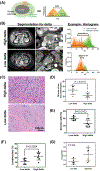
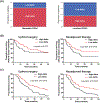
Results: In localized and metastatic PDAC, quantifying the change in enhancement on CT scans at the interface between tumor and parenchyma (delta) demonstrated that patients with conspicuous (high-delta) tumors had significantly less stroma, higher likelihood of multiple common pathway mutations, more mesenchymal features, higher likelihood of early distant metastasis, and shorter survival times compared with those with inconspicuous (low-delta) tumors. Pathologic measurements of stromal and mesenchymal features of the tumors supported the mathematical model's underlying theory for PDAC growth.
Conclusions: At baseline diagnosis, a visually striking and quantifiable CT imaging feature reflects the molecular and pathological heterogeneity of PDAC, and may be used to stratify patients into distinct subtypes. Moreover, growth patterns of PDAC may be described using physical principles, enabling new insights into diagnosis and treatment of this deadly disease.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Hidalgo M Pancreatic cancer. The New England journal of medicine 2010;362(17):1605–17 doi 10.1056/NEJMra0901557. - DOI - PubMed
-
- Koay EJ, Truty MJ, Cristini V, Thomas RM, Chen R, Chatterjee D, et al. Transport properties of pancreatic cancer describe gemcitabine delivery and response. The Journal of clinical investigation 2014;124(4):1525–36 doi 10.1172/JCI73455. - DOI - PMC - PubMed
-
- Ferrari M Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends in biotechnology 2010;28(4):181–8 doi 10.1016/j.tibtech.2009.12.007. - DOI - PMC - PubMed
-
- Pascal J, Bearer EL, Wang Z, Koay EJ, Curley SA, Cristini V. Mechanistic patient-specific predictive correlation of tumor drug response with microenvironment and perfusion measurements. Proceedings of the National Academy of Sciences of the United States of America 2013;110(35):14266–71 doi 10.1073/pnas.1300619110. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- U01 CA200468/CA/NCI NIH HHS/United States
- U01 CA213759/CA/NCI NIH HHS/United States
- U54 CA210181/CA/NCI NIH HHS/United States
- R01 CA218004/CA/NCI NIH HHS/United States
- U54 CA151668/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U54 CA143837/CA/NCI NIH HHS/United States
- R01 CA226537/CA/NCI NIH HHS/United States
- U01 CA196403/CA/NCI NIH HHS/United States
- P30 CA062203/CA/NCI NIH HHS/United States
- R01 CA221971/CA/NCI NIH HHS/United States
- T32 CA009599/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- R01 CA222007/CA/NCI NIH HHS/United States
- P50 GM076516/GM/NIGMS NIH HHS/United States
- U54 CA217378/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

