Outcome of First-Line Systemic Treatment for Unresectable Conventional, Dedifferentiated, Mesenchymal, and Clear Cell Chondrosarcoma
- PMID: 30082492
- PMCID: PMC6324625
- DOI: 10.1634/theoncologist.2017-0574
Outcome of First-Line Systemic Treatment for Unresectable Conventional, Dedifferentiated, Mesenchymal, and Clear Cell Chondrosarcoma
Abstract
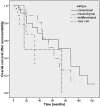
Background: Chondrosarcoma is a heterogeneous group of primary bone sarcoma with an excellent overall survival after local therapy. However, the small percentage of patients who have no surgical treatment options have a very poor prognosis. We retrospectively collected data from these patients in four sarcoma centers and compared the progression-free survival (PFS) for the different treatment regimens used for the four chondrosarcoma subtypes.
Materials and methods: Patients diagnosed with unresectable chondrosarcoma in all four major sarcoma centers were included, and data on first-line systemic therapy were retrospectively collected for analysis.
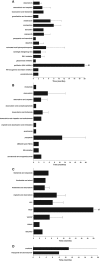
Results: A total of 112 patients were enrolled in this retrospective analysis: 50 conventional, 25 mesenchymal, 34 dedifferentiated, and 3 clear cell chondrosarcoma patients. In conventional chondrosarcoma patients, the longest mean PFS (6.7 months) was found in the group treated with antihormonal therapy. Patients diagnosed with mesenchymal chondrosarcoma were all treated with multidrug chemotherapy, and the mean PFS was 6.7 months. Doxorubicin monotherapy seems to have an unexplained better PFS than doxorubicin-based combination therapy in patients with dedifferentiated chondrosarcoma (5.5 vs. 2.8 months, respectively; p = .275).
Conclusion: Prospective studies need to be conducted based on preclinical work to develop a uniform regimen to treat advanced chondrosarcoma patients according to the diagnosed subtype and improve survival.
Implications for practice: Currently, there are no uniform treatment lines for advanced chondrosarcoma patients, which results in a very diverse group of treatment regimens being used. In this study, the data of 112 patients was collected. It was concluded that some treatment regimens seem to have a better progression-free survival compared with others, and that these results also differ between the chondrosarcoma subtypes. Prospective studies need to be conducted based on preclinical work to develop a uniform regimen to treat advanced chondrosarcoma patients according to the diagnosed histological subtype to improve their survival.
Keywords: Chondrosarcoma; Progression‐free survival; Retrospective; Systemic treatment; Unresectable.
© AlphaMed Press 2018.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

References
-
- Fletcher CDM, Bridge JA, Hogendoorn PC. et al., eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed Lyon, France: IARC Press, 2013:264.
-
- Cesari M, Bertoni F, Bacchini P et al. Mesenchymal chondrosarcoma. An analysis of patients treated at a single institution. Tumori 2007;93:423–427. - PubMed
-
- Dantonello TM, Int‐Veen C, Leuschner I et al. Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: Experiences of the CWS and COSS study groups. Cancer 2008;112:2424–2431. - PubMed
-
- Grimer RJ, Gosheger G, Taminiau A et al. Dedifferentiated chondrosarcoma: Prognostic factors and outcome from a European group. Eur J Cancer 2007;43:2060–2065. - PubMed
-
- Staals EL, Bacchini P, Bertoni F. Dedifferentiated central chondrosarcoma. Cancer 2006;106:2682–2691. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

