Drosophila melanogaster as a function-based high-throughput screening model for antinephrolithiasis agents in kidney stone patients
- PMID: 30082495
- PMCID: PMC6262805
- DOI: 10.1242/dmm.035873
Drosophila melanogaster as a function-based high-throughput screening model for antinephrolithiasis agents in kidney stone patients
Abstract
Kidney stone disease involves the aggregation of stone-forming salts consequent to solute supersaturation in urine. The development of novel therapeutic agents for this predominantly metabolic and biochemical disorder have been hampered by the lack of a practical pre-clinical model amenable to drug screening. Here, Drosophila melanogaster, an emerging model for kidney stone disease research, was adapted as a high-throughput functional drug screening platform independent of the multifactorial nature of mammalian nephrolithiasis. Through functional screening, the therapeutic potential of a novel compound commonly known as arbutin that specifically binds to oxalate, a key component of kidney calculi, was identified. Through isothermal titration calorimetry, high-performance liquid chromatography and atomic force microscopy, arbutin was determined to interact with calcium and oxalate in both free and bound states, disrupting crystal lattice structure, growth and crystallization. When used to treat patient urine samples, arbutin significantly abrogated calculus formation in vivo and outperformed potassium citrate in low pH urine conditions, owing to its oxalate-centric mode of action. The discovery of this novel antilithogenic compound via D. melanogaster, independent of a mammalian model, brings greater recognition to this platform, for which metabolic features are primary outcomes, underscoring the power of D. melanogaster as a high-throughput drug screening platform in similar disorders. This is the first description of the use of D. melanogaster as the model system for a high-throughput chemical library screen. This article has an associated First Person interview with the first authors of the paper.
Keywords: Calcium oxalate; Drosophila melanogaster; Fluorescent bisphosphonate; Intravital imaging; Nephrolithiasis.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
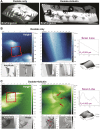
Figures






References
-
- Balcke P., Schmidt P., Zazgornik J., Kopsa H. and Minar E. (1983). Pyridoxine therapy in patients with renal calcium oxalate calculi. Proc. Eur. Dial. Transplant Assoc. 20, 417-421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

