The rise of neutron cryo-crystallography
- PMID: 30082515
- PMCID: PMC6079629
- DOI: 10.1107/S205979831800640X
The rise of neutron cryo-crystallography
Abstract
The use of boiled-off liquid nitrogen to maintain protein crystals at 100 K during X-ray data collection has become almost universal. Applying this to neutron protein crystallography offers the opportunity to significantly broaden the scope of biochemical problems that can be addressed, although care must be taken in assuming that direct extrapolation to room temperature is always valid. Here, the history to date of neutron protein cryo-crystallography and the particular problems and solutions associated with the mounting and cryocooling of the larger crystals needed for neutron crystallography are reviewed. Finally, the outlook for further cryogenic neutron studies using existing and future neutron instrumentation is discussed.
Keywords: cryogenic data collection; enzyme mechanisms; neutron crystallography.
open access.
Figures
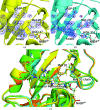

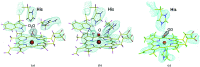

 and
and  , respectively. The red crosses show alternative structures eliminated by NPX.
, respectively. The red crosses show alternative structures eliminated by NPX.References
-
- Bernal, J. D. & Crowfoot, D. (1934). Nature (London), 133, 794–795.
-
- Blakeley, M. P. (2009). Crystallogr. Rev. 15, 157–218.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

