Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C-H bonds
- PMID: 30082883
- PMCID: PMC6650386
- DOI: 10.1038/s41557-018-0087-7
Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C-H bonds
Abstract
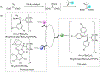
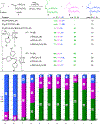
C-H functionalization represents a promising approach for the synthesis of complex molecules. Instead of relying on modifying the functional groups present in a molecule, the synthetic sequence is achieved by carrying out selective reactions on the C-H bonds, which traditionally would have been considered to be the unreactive components of a molecule. A major challenge is to design catalysts to control both the site- and stereoselectivity of the C-H functionalization. We have been developing dirhodium catalysts with different selectivity profiles in C-H functionalization reactions with donor/acceptor carbenes as reactive intermediates. Here we describe a new dirhodium catalyst capable of the functionalization of non-activated primary C-H bonds with high levels of site selectivity and enantioselectivity.
Figures




References
-
- Godula K & Sames D, C–H bond functionalization in complex organic synthesis. Science 312, 67–72 (2006). - PubMed
-
- Gutekunst WR & Baran PS, C–H functionalization logic in total synthesis. Chem. Soc. Rev. 40, 1976–1991 (2011). - PubMed
-
- Wencel-Delord J & Glorius F, C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 5, 369–375 (2013). - PubMed
-
- Noisier AF & Brimble MA, C–H functionalization in the synthesis of amino acids and peptides. Chem. Rev. 114, 8775–8806 (2014). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

