Tigecycline as a dual inhibitor of retinoblastoma and angiogenesis via inducing mitochondrial dysfunctions and oxidative damage
- PMID: 30082885
- PMCID: PMC6079108
- DOI: 10.1038/s41598-018-29938-x
Tigecycline as a dual inhibitor of retinoblastoma and angiogenesis via inducing mitochondrial dysfunctions and oxidative damage
Abstract
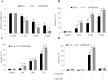
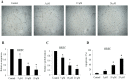
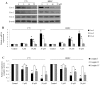
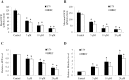
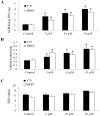
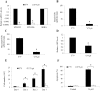
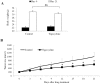
Retinoblastoma is the most common intraocular malignancy in children with poor prognosis. The progression of retinoblastoma is dependent on a robust angiogenic response. Targeting both retinoblastoma cells and angiogenesis may therefore provide an alternative therapeutic strategy in retinoblastoma. Here, we demonstrated the inhibitory effects of tigecycline, a FDA-approved antibiotic, in retinoblastoma and angiogenesis in vitro and in vivo. We showed that tigecycline significantly inhibited growth and induced caspase-dependent apoptosis of multiple retinoblastoma cell lines. Tigecycline also effectively inhibited angiogenesis through suppressing capillary network formation, migration, proliferation and survival of human retinal microvascular endothelial cell (HREC). Mechanistically, tigecycline acts on both retinoblastoma cells and HREC via inhibiting mitochondrial protein translation, resulting in mitochondrial dysfunction, energy crisis, and oxidative damage. Importantly, we demonstrated the in vivo efficacy of tigecycline in inhibiting retinoblastoma and angiogenesis, and inducing oxidative stress on xenograft mouse model. In addition, ATP levels and growth rates were largely affected in retinoblastoma ρ0 cells that lacked mitochondrial respiration. Our work provides systematic pre-clinical evidence for repurposing tigecycline from its traditional use for retinoblastoma treatment. Our work demonstrates the essential roles of mitochondrial metabolism in both retinoblastoma and its angiogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Biological Functions and Molecular Mechanisms of Antibiotic Tigecycline in the Treatment of Cancers.Int J Mol Sci. 2019 Jul 22;20(14):3577. doi: 10.3390/ijms20143577. Int J Mol Sci. 2019. PMID: 31336613 Free PMC article. Review.
-
Antibiotic tigecycline enhances cisplatin activity against human hepatocellular carcinoma through inducing mitochondrial dysfunction and oxidative damage.Biochem Biophys Res Commun. 2017 Jan 29;483(1):17-23. doi: 10.1016/j.bbrc.2017.01.021. Epub 2017 Jan 6. Biochem Biophys Res Commun. 2017. PMID: 28069382
-
Antibiotic bedaquiline effectively targets growth, survival and tumor angiogenesis of lung cancer through suppressing energy metabolism.Biochem Biophys Res Commun. 2018 Jan 1;495(1):267-272. doi: 10.1016/j.bbrc.2017.10.136. Epub 2017 Oct 28. Biochem Biophys Res Commun. 2018. PMID: 29107691
-
Inhibition of mitochondrial respiration by tigecycline selectively targets thyroid carcinoma and increases chemosensitivity.Clin Exp Pharmacol Physiol. 2019 Oct;46(10):890-897. doi: 10.1111/1440-1681.13126. Epub 2019 Jul 31. Clin Exp Pharmacol Physiol. 2019. PMID: 31209921
-
[Anti-tumor activity of tigecycline: a review].Sheng Wu Gong Cheng Xue Bao. 2021 Sep 25;37(9):3031-3041. doi: 10.13345/j.cjb.200630. Sheng Wu Gong Cheng Xue Bao. 2021. PMID: 34622615 Review. Chinese.
Cited by
-
Third-Generation Tetracyclines: Current Knowledge and Therapeutic Potential.Biomolecules. 2024 Jun 30;14(7):783. doi: 10.3390/biom14070783. Biomolecules. 2024. PMID: 39062497 Free PMC article. Review.
-
Unraveling the Anti-Cancer Mechanisms of Antibiotics: Current Insights, Controversies, and Future Perspectives.Antibiotics (Basel). 2024 Dec 25;14(1):9. doi: 10.3390/antibiotics14010009. Antibiotics (Basel). 2024. PMID: 39858295 Free PMC article. Review.
-
Biological Functions and Molecular Mechanisms of Antibiotic Tigecycline in the Treatment of Cancers.Int J Mol Sci. 2019 Jul 22;20(14):3577. doi: 10.3390/ijms20143577. Int J Mol Sci. 2019. PMID: 31336613 Free PMC article. Review.
-
New acorane-sesequiterpenes and anti-retinoblastoma constituents from the marine algicolous fungus Trichoderma harzianum NTU2180 guided by molecular networking strategy.Bot Stud. 2025 Jan 14;66(1):2. doi: 10.1186/s40529-024-00449-5. Bot Stud. 2025. PMID: 39808245 Free PMC article.
-
Overcoming Glucocorticoid Resistance in Acute Lymphoblastic Leukemia: Repurposed Drugs Can Improve the Protocol.Front Oncol. 2021 Mar 11;11:617937. doi: 10.3389/fonc.2021.617937. eCollection 2021. Front Oncol. 2021. PMID: 33777761 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

