Relative Efficacy of Checkpoint Inhibitors for Advanced NSCLC According to Programmed Death-Ligand-1 Expression: A Systematic Review and Network Meta-Analysis
- PMID: 30082893
- PMCID: PMC6078964
- DOI: 10.1038/s41598-018-30277-0
Relative Efficacy of Checkpoint Inhibitors for Advanced NSCLC According to Programmed Death-Ligand-1 Expression: A Systematic Review and Network Meta-Analysis
Abstract
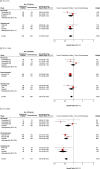
Although currently available immune checkpoint inhibitors with similar but slightly different indications are recommended for patients with advanced non-small cell lung cancer (NSCLC), their effects by programmed death-ligand-1 (PD-L1) expression level are not yet known. This meta-analysis aims to assess the survival benefit and comparative efficacy of checkpoint inhibitors according to PD-L1 expression level: <1%, 1-49%, and ≥50%. We searched the MEDLINE, EMBASE, and Cochrane database through December 2017. A fixed-effect Bayesian network meta-analysis (NMA) was performed to estimate hazard ratios (HRs) for overall survival (OS) with 95% credible intervals (CrIs). Seven trials including 3688 patients were selected from among the 673 screened studies. Checkpoint inhibitor remarkably improved OS over chemotherapy in the PD-L1 ≥ 50% subgroup compared with the PD-L1 < 1% and PD-L1 1-49% subgroups. Atezolizumab, nivolumab, and nivolumab were the most effective agents for second- or later-line settings in the PD-L1 < 1%, PD-L1 1-49%, and PD-L1 ≥ 50% subgroups, respectively. PD-L1 expression ≥50% on tumor cells could be a reliable indicator that helps patient selection in view of cost-efficiency, and each checkpoint inhibitor reported to be the best agent by PD-L1 expression level could be carefully recommended in each PD-L1 expression subgroup.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

