Pharmacokinetics and Bioavailability Study of a Prednisolone Tablet as a Single Oral Dose in Bangladeshi Healthy Volunteers
- PMID: 30083083
- PMCID: PMC6073839
- DOI: 10.1177/1559325818783932
Pharmacokinetics and Bioavailability Study of a Prednisolone Tablet as a Single Oral Dose in Bangladeshi Healthy Volunteers
Abstract
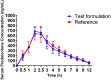
The aim of the study was to assess the pharmacokinetic and bioavailability of 2 formulations of 5-mg prednisolone tablets, reference product (Teva UK Limited) and Pred (Eskayef Bangladesh Ltd) as test product. The open-label, randomized, 2-way crossover studies were conducted on 14 healthy subjects. Participants were assigned to receive both products as a single dose (20 mg formulations, 4 × 5 mg tablets) followed by a 2 weeks' washout period. Following oral administration, samples were obtained at various time intervals and analyzed for prednisolone concentrations using a validated high-performance liquid chromatography assay method with ultraviolet detection. The obtained values for test and reference products were 683.00 ± 94.54 ng/mL and 635.16 ± 125.57 ng/mL for Cmax; 2716.54 ± 196.28 ng·h/mL and 2780.5 ± 119.73 ng·h/mL for AUC0-12; 3284.36 ± 138.12 ng·h/mL and 3317.96 ± 133.95 ng·h/mL for AUC0-∞, respectively. From the paired Student t test, no significant differences between 2 formulations were observed (P > .05). The 90% confidence intervals of Cmax, AUC0-12, and AUC0-∞ were found to be 99.0% to 100.9%, 99.4% to 100.5%, and 99.9% to 101.3%, respectively. Finally, it can be concluded that Pred (Test) of Eskayef Bangladesh Ltd and prednisolone (Reference) of Teva UK Limited are bioequivalent and interchangeable.
Keywords: Bangladeshi healthy volunteers.; bioavailability; bioequivalence; pharmacokinetics; prednisolone.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Vogt M, Derendorf H, Krämer J, et al. Biowaiver monographs for immediate release solid oral dosage forms: prednisolone. J Pharm Sci. 2007;96(1):27–37. doi:10.1002/jps.20768. - PubMed
-
- 20th World Health Organization Model List of Essential Medicine March. 2007. http://www.who.int/medicines/publications/essentialmedicines/20th_EML201.... Accessed June 13, 2018.
-
- Stahn C, Löwenberg M, Hommes DW, Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275(1-2):71–78. doi:10.1016/j.mce.2007.05.019 - PubMed
-
- Tanner A, Bochner F, Caffin J, Halliday J, Powell L. Dose-dependent prednisolone kinetics. Clin Pharmacol Ther. 1979;25(5 pt 1):571–578. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases