Glutamine supplementation improves intestinal cell proliferation and stem cell differentiation in weanling mice
- PMID: 30083086
- PMCID: PMC6060183
- DOI: 10.29219/fnr.v62.1439
Glutamine supplementation improves intestinal cell proliferation and stem cell differentiation in weanling mice
Abstract
Background: Intestinal stem cells can be differentiated into absorptive enterocytes and secretory cells, including Paneth cells, goblet cells, and enteroendocrine cells. Glutamine is a primary metabolic fuel of small intestinal enterocytes and is essential for the viability and growth of intestinal cells.
Objective: Whether glutamine supplementation affects the differentiation of intestinal stem cells is unknown.
Design: Three-week-old ICR (Institute of Cancer Research) male mice were divided randomly into two groups: 1) mice receiving a basal diet and normal drinking water and 2) mice receiving a basal diet and drinking water supplemented with glutamine. After 2 weeks, the mice were sacrificed to collect the ileum for analysis.
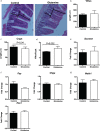
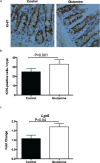
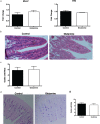
Results: The study found that glutamine supplementation in weanling mice decreases the crypt depth in the ileum, leading to higher ratio of villus to crypt in the ileum, but promotes cell proliferation of intestinal cells and mRNA expression of Lgr5 (leucine-rich repeat-containing g-protein coupled receptor5) in the ileum. Glutamine has no effect on the number of Paneth cells and goblet cells, and the expression of markers for absorptive enterocytes, Paneth cells, goblet cells, and enteroendocrine cells.
Conclusion: These findings reveal the beneficial effects of dietary glutamine supplementation to improve intestinal morphology in weanling mammals.
Keywords: Paneth cells; glutamine; goblet cells; intestinal stem cells; weaning.
Figures


References
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449(7165): 1003–7. - PubMed
-
- Peng HS, Pooyaiah N, Forrester M, Cochran E, Wang Q. Ex vivo culture of primary intestinal stem cells in collagen gels and foams. ACS Biomater Sci Eng 2015; 1(1): 37–42. - PubMed
-
- Bloemendaal AL, Buchs NC, George BD, Guy RJ. Intestinal stem cells and intestinal homeostasis in health and in inflammation: a review. Surgery 2016; 159(5): 1237–48. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

