Interventional Potential of Recombinant Feline Hepatocyte Growth Factor in a Mouse Model of Non-alcoholic Steatohepatitis
- PMID: 30083132
- PMCID: PMC6064873
- DOI: 10.3389/fendo.2018.00378
Interventional Potential of Recombinant Feline Hepatocyte Growth Factor in a Mouse Model of Non-alcoholic Steatohepatitis
Abstract
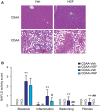
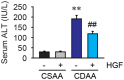
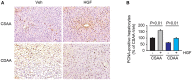
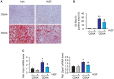
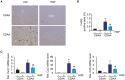
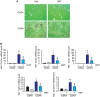
Background and Aims: Hepatocyte growth factor (HGF) is a multifunctional pleiotropic protein involved in tissue regeneration, protection, angiogenesis, anti-inflammatory and anti-fibrotic responses, and tumorigenesis, through binding to its receptor MET. Recombinant HGF protein has been shown to mitigate various liver disease models, such as alcohol-induced liver injury, hepatic ischemia-reperfusion injury, and fibrosis. This study aimed to investigate the anti-inflammatory, anti-fibrotic, and anti-lipogenic effects of exogenous administration of feline HGF on a non-alcoholic steatohepatitis (NASH) mouse model. Methods: Wild-type C57BL/6 mice were fed a choline-deficient amino acid defined (CDAA) diet for 3 weeks to create the mouse model of NASH, which displays hepatic steatosis, inflammation, injury, and very mild fibrosis. One mg/kg of recombinant feline HGF was administered intravenously daily in the last 7 days of the total 3 weeks of CDAA diet feeding. Then, hepatic steatosis, inflammation, injury, and fibrogenic gene expression was examined. Results: After 3 weeks of a CDAA diet-feeding, the vehicle-treated mice exhibited evident deposition of lipid droplets in hepatocytes, inflammatory cell infiltration, and hepatocyte ballooning along with increased serum ALT levels whereas recombinant HGF-treated mice showed reduced hepatic steatosis, inflammation, and ballooned hepatocytes with a reduction of serum ALT levels. Recombinant HGF administration promoted hepatocyte proliferation. Increased hepatic lipid accumulation was accompanied by elevated expression of lipogenesis genes Fasn and Dgat1 in vehicle-treated mice. In HGF-treated mice, these genes were reduced with a decrease of lipid accumulation in the liver. Consistent with the anti-inflammatory property of HGF, augmented macrophage infiltration and upregulation of chemokines, Cxcl1, Ccl2, and Ccl5 in the CDAA diet fed mice, were suppressed by the addition of the HGF treatment. Finally, we examined the fibrotic response. The vehicle-treated mice had mild fibrosis with upregulation of Col1a1, Acta2, Timp1, Tgfb1, and Serpine1 expression. Recombinant HGF treatment significantly suppressed fibrogenic gene expression and collagen deposition in the liver. Conclusion: Recombinant feline HGF treatment suppressed the progression of NASH in a CDAA diet feeding mouse model.This suggests that recombinant HGF protein has therapeutic potential for NASH.
Keywords: HGF; NAFLD; NASH; inflammation; recombinant.
Figures
Similar articles
-
Inhibition of hyaluronan synthesis by 4-methylumbelliferone ameliorates non-alcoholic steatohepatitis in choline-deficient L-amino acid-defined diet-induced murine model.Arch Pharm Res. 2021 Feb;44(2):230-240. doi: 10.1007/s12272-021-01309-7. Epub 2021 Jan 24. Arch Pharm Res. 2021. PMID: 33486695 Free PMC article.
-
TRIF Differentially Regulates Hepatic Steatosis and Inflammation/Fibrosis in Mice.Cell Mol Gastroenterol Hepatol. 2017 Jan 17;3(3):469-483. doi: 10.1016/j.jcmgh.2016.12.004. eCollection 2017 May. Cell Mol Gastroenterol Hepatol. 2017. PMID: 28462384 Free PMC article.
-
Transforming growth factor beta signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism in mice.Hepatology. 2014 Feb;59(2):483-95. doi: 10.1002/hep.26698. Epub 2013 Dec 18. Hepatology. 2014. PMID: 23996730 Free PMC article.
-
Pathogenesis of Non-alcoholic Steatohepatitis and Its Potential Therapeutic Strategies.In: Nakao K, Minato N, Uemoto S, editors. Innovative Medicine: Basic Research and Development [Internet]. Tokyo: Springer; 2015. In: Nakao K, Minato N, Uemoto S, editors. Innovative Medicine: Basic Research and Development [Internet]. Tokyo: Springer; 2015. PMID: 29787166 Free Books & Documents. Review.
-
HGF-Met Pathway in Regeneration and Drug Discovery.Biomedicines. 2014 Oct 31;2(4):275-300. doi: 10.3390/biomedicines2040275. Biomedicines. 2014. PMID: 28548072 Free PMC article. Review.
Cited by
-
Liver Fibrosis-From Mechanisms of Injury to Modulation of Disease.Front Med (Lausanne). 2022 Jan 11;8:814496. doi: 10.3389/fmed.2021.814496. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35087852 Free PMC article. Review.
-
Inhibition of hyaluronan synthesis by 4-methylumbelliferone ameliorates non-alcoholic steatohepatitis in choline-deficient L-amino acid-defined diet-induced murine model.Arch Pharm Res. 2021 Feb;44(2):230-240. doi: 10.1007/s12272-021-01309-7. Epub 2021 Jan 24. Arch Pharm Res. 2021. PMID: 33486695 Free PMC article.
-
Designing receptor agonists with enhanced pharmacokinetics by grafting macrocyclic peptides into fragment crystallizable regions.Nat Biomed Eng. 2023 Feb;7(2):164-176. doi: 10.1038/s41551-022-00955-6. Epub 2022 Nov 7. Nat Biomed Eng. 2023. PMID: 36344661 Free PMC article.
-
A cyclic peptide-grafted Fc with hepatocyte growth factor functionality ameliorates hepatic fibrosis in a non-alcoholic steatohepatitis mouse model.iScience. 2024 Jul 2;27(8):110426. doi: 10.1016/j.isci.2024.110426. eCollection 2024 Aug 16. iScience. 2024. PMID: 39108737 Free PMC article.
-
Exploring the Gamut of Receptor Tyrosine Kinases for Their Promise in the Management of Non-Alcoholic Fatty Liver Disease.Biomedicines. 2021 Nov 26;9(12):1776. doi: 10.3390/biomedicines9121776. Biomedicines. 2021. PMID: 34944593 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

