The Role of Efferocytosis in Autoimmune Diseases
- PMID: 30083153
- PMCID: PMC6064952
- DOI: 10.3389/fimmu.2018.01645
The Role of Efferocytosis in Autoimmune Diseases
Abstract
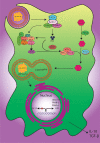
Apoptosis happens continuously for millions of cells along with the active removal of apoptotic debris in order to maintain tissue homeostasis. In this respect, efferocytosis, i.e., the process of dead cell clearance, is orchestrated through cell exposure of a set of "find me," "eat me," and "tolerate me" signals facilitating the engulfment of dying cells through phagocytosis by macrophages and dendritic cells. The clearance of dead cells via phagocytes is of utmost importance to maintain the immune system tolerance to self-antigens. Accordingly, this biological activity prevents the release of autoantigens by dead cells, thus potentially suppressing the undesirable autoreactivity of immune cells and the appearance of inflammatory autoimmune disorders as systemic lupus erythematous and rheumatoid arthritis. In the present study, the apoptosis pathways and their immune regulation were reviewed. Moreover, efferocytosis process and its impairment in association with some autoimmune diseases were discussed.
Keywords: apoptosis; autoimmune disease; efferocytosis; phagocytosis; systemic lupus erythematous.
Figures

References
-
- Tanaka M, Nishitai G. Immune regulation by dead cell clearance. Curr Top Microbiol Immunol (2017) 403:171–83. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

