Non-thermal ablation of non-neoplastic Barrett's esophagus with the novel EndoRotor® resection device
- PMID: 30083329
- PMCID: PMC6068777
- DOI: 10.1177/2050640618758214
Non-thermal ablation of non-neoplastic Barrett's esophagus with the novel EndoRotor® resection device
Abstract
Background: International guidelines suggest endoscopic resection for all patients with low-risk mucosal cancer. Ultimately, it is essential to treat the remaining Barrett's esophagus as part of the treatment. Different thermal ablative therapies have been implemented to effect this treatment. They can lead to potential post-therapeutic stenosis. Furthermore, a histologic assessment of treated mucosa is not possible.
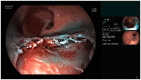
Objective: Clinical evaluation of a novel, non-thermal resection device (EndoRotor®) in the treatment of non-neoplastic Barrett's esophagus was conducted.
Methods: Fourteen patients with early Barrett's carcinoma were treated with endoscopic resection. Subsequently, EndoRotor® therapy was performed for resection of the remaining Barrett's mucosa. Complications were assessed during the study. After a three-month period patients received follow-up endoscopy to evaluate post-therapeutic stenosis.
Results: On average, 674 mm2 (172 mm2 - 1600 mm2) of Barrett's mucosa was treated with the novel device. In six (37.5%) cases, intra-procedural bleeding occurred with the need for hemostasis. All bleeding could be managed by endoscopic therapy alone. After a three-month follow-up there was no post-therapeutic stenosis registered.
Conclusion: EndoRotor® resection is a feasible non-thermal treatment of non-neoplastic Barrett's esophagus. Larger trials have to evaluate risks and benefits of this novel device.
Keywords: Barrett’s cancer; Esophageal cancer; ablation; adenocarcinoma; athermal; intestinal metaplasia.
Figures



References
-
- Wani S, Mathur SC, Curvers WL, et al. Greater interobserver agreement by endoscopic mucosal resection than biopsy samples in Barrett’s dysplasia. Clin Gastroenterol Hepatol 2010; 8: 783–788. - PubMed
-
- Overholt B, Panjehpour M, Tefftellar E, et al. Photodynamic therapy for treatment of early adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc 1993; 39: 73–76. - PubMed
-
- Manner H, May A, Miehlke S, et al. Ablation of nonneoplastic Barrett’s mucosa using argon plasma coagulation with concomitant esomeprazole therapy (APBANEX): A prospective multicenter evaluation. Am J Gastroenterol 2006; 101: 1762–1769. - PubMed
-
- Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett’s epithelium after endoscopic resection: A randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014; 46: 6–12. - PubMed
-
- Ganz RA, Utley DS, Stern RA, et al. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: A phased evaluation in the porcine and in the human esophagus. Gastrointest Endosc 2004; 60: 1002–1010. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

