Radical-Type Reactivity and Catalysis by Single-Electron Transfer to or from Redox-Active Ligands
- PMID: 30084211
- PMCID: PMC6471147
- DOI: 10.1002/chem.201802606
Radical-Type Reactivity and Catalysis by Single-Electron Transfer to or from Redox-Active Ligands
Abstract
Controlled ligand-based redox-activity and chemical non-innocence are rapidly gaining importance for selective (catalytic) processes. This Concept aims to provide an overview of the progress regarding ligand-to-substrate single-electron transfer as a relatively new mode of operation to exploit ligand-centered reactivity and catalysis based thereon.
Keywords: non-innocent ligands; radical; redox-active ligands; single-electron transfer; substrate.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

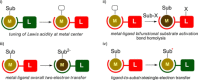


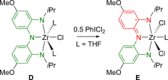





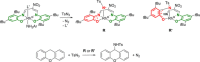

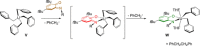
References
-
- None
-
- Broere D. L. J., Plessius R., van der Vlugt J. I., Chem. Soc. Rev. 2015, 44, 6886; - PubMed
-
- Luca O. R., Crabtree R. H., Chem. Soc. Rev. 2013, 42, 1440–1459; - PubMed
-
- Praneeth V. K. K., Ringenberg M. R., Ward T. R., Angew. Chem. Int. Ed. 2012, 51, 10228–10234; - PubMed
- Angew. Chem. 2012, 124, 10374–10380;
-
- Lyaskovskyy V., de Bruin B., ACS Catal. 2012, 2, 270–279;
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

