Capillary and Venous Blood Glucose Accuracy in Blood Glucose Meters Versus Reference Standards: The Impact of Study Design on Accuracy Evaluations
- PMID: 30084263
- PMCID: PMC6501528
- DOI: 10.1177/1932296818790228
Capillary and Venous Blood Glucose Accuracy in Blood Glucose Meters Versus Reference Standards: The Impact of Study Design on Accuracy Evaluations
Abstract
Background: Anecdotal blood glucose assessments conducted by health care professionals (HCPs) in the field have highlighted differences in results when methodology used is not according to best practices for measuring blood glucose. This study assessed the impact on accuracy of blood glucose measurements when methodology deviates from the recommended study design and recommended reference instrument.
Methods: Adults with type 1 or type 2 diabetes provided capillary and venous blood samples for accuracy assessments using OneTouch® Verio® (Verio) and OneTouch® Ultra 2® (Ultra) blood glucose meters (BGM) and two different reference instruments.
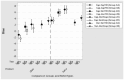
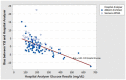
Results: Increases in mean bias were observed when comparing capillary to venous samples tested on the BGMs and the recommended reference instrument. Mean bias was even greater when a hospital blood glucose analyzer was used to measure venous plasma glucose. Increases in mean bias observed for Ultra BGM when testing venous blood on the meter compared to the recommended reference instrument was likely due to the interfering effects of low oxygen levels in the venous blood sample. Conversely, Verio meters, which are insensitive to low oxygen levels, showed little difference from baseline when testing venous blood on the meter compared to results from the same venous sample measured on a reference instrument.
Conclusions: Deviations from the best practice study design of comparing capillary blood glucose results tested on the blood glucose meter with the manufacturer's stated reference instrument will affect accuracy of blood glucose measurements.
Keywords: Ultra; Verio; YSI; blood glucose accuracy; blood glucose monitor.
Conflict of interest statement
Figures

Comment in
-
Analysis of "Capillary and Venous Blood Glucose Accuracy in Blood Glucose Meters Versus Reference Standards: The Impact of Study Design on Accuracy Evaluations".J Diabetes Sci Technol. 2019 May;13(3):553-558. doi: 10.1177/1932296818799365. Epub 2018 Sep 15. J Diabetes Sci Technol. 2019. PMID: 30222000 Free PMC article.
-
Why the Details of Glucose Meter Evaluations Matters.J Diabetes Sci Technol. 2019 May;13(3):559-560. doi: 10.1177/1932296818803113. Epub 2018 Oct 17. J Diabetes Sci Technol. 2019. PMID: 30328716 Free PMC article.
References
-
- Mahoney JJ, Ellison JM. Assessing glucose monitor performance—a standardized approach. Diabetes Technol Ther. 2007;9:545-552. - PubMed
-
- Demircik F, Klonoff D, Musholt PB, Ramljak S, Pfützner A. Successful performance of laboratory investigations with blood glucose meters employing a dynamic electrochemistry-based correction algorithm is dependent on careful sample handling. Diabetes Technol Ther. 2016;18:650-656. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

