Neuroarchitecture of the Drosophila central complex: A catalog of nodulus and asymmetrical body neurons and a revision of the protocerebral bridge catalog
- PMID: 30084503
- PMCID: PMC6283239
- DOI: 10.1002/cne.24512
Neuroarchitecture of the Drosophila central complex: A catalog of nodulus and asymmetrical body neurons and a revision of the protocerebral bridge catalog
Abstract
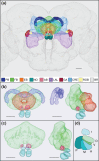
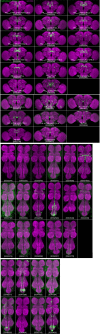
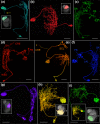
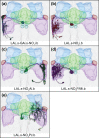
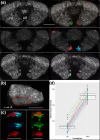
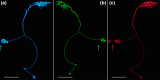
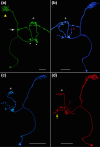
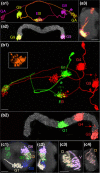
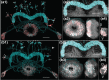
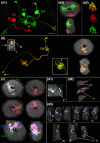
The central complex, a set of neuropils in the center of the insect brain, plays a crucial role in spatial aspects of sensory integration and motor control. Stereotyped neurons interconnect these neuropils with one another and with accessory structures. We screened over 5,000 Drosophila melanogaster GAL4 lines for expression in two neuropils, the noduli (NO) of the central complex and the asymmetrical body (AB), and used multicolor stochastic labeling to analyze the morphology, polarity, and organization of individual cells in a subset of the GAL4 lines that showed expression in these neuropils. We identified nine NO and three AB cell types and describe them here. The morphology of the NO neurons suggests that they receive input primarily in the lateral accessory lobe and send output to each of the six paired noduli. We demonstrate that the AB is a bilateral structure which exhibits asymmetry in size between the left and right bodies. We show that the AB neurons directly connect the AB to the central complex and accessory neuropils, that they target both the left and right ABs, and that one cell type preferentially innervates the right AB. We propose that the AB be considered a central complex neuropil in Drosophila. Finally, we present highly restricted GAL4 lines for most identified protocerebral bridge, NO, and AB cell types. These lines, generated using the split-GAL4 method, will facilitate anatomical studies, behavioral assays, and physiological experiments.
Keywords: AB_1549585; AB_1625981; AB_2314866; AB_915420; Drosophila brain; GAL4; MCFO; asymmetrical body; central complex; nodulus; protocerebral bridge.
© 2018 The Authors. The Journal of Comparative Neurology published by Wiley Periodicals, Inc.
Figures



References
-
- Baker, D. A. , Beckingham, K. M. , & Armstrong, J. D. (2007). Functional dissection of the neural substrates for gravitaxic maze behavior in Drosophila melanogaster . The Journal of Comparative Neurology, 501(5), 756–764. - PubMed
-
- Bidaye, S. S. , Machacek, C. , Wu, Y. , & Dickson, B. J. (2014). Neuronal control of Drosophila walking direction. Science, 344(6179), 97–101. - PubMed
-
- Bouhouche, A. , Vaysse, G. , & Corbiere, M. (1993). Immunocytochemical and learning studies of a Drosophila melanogaster neurological mutant, no‐bridgeKS49 as an approach to the possible role of the central complex. Journal of Neurogenetics, 9(2), 105–121. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

