Testing a Multivariate Proteomic Panel for Traumatic Brain Injury Biomarker Discovery: A TRACK-TBI Pilot Study
- PMID: 30084741
- PMCID: PMC6306686
- DOI: 10.1089/neu.2017.5449
Testing a Multivariate Proteomic Panel for Traumatic Brain Injury Biomarker Discovery: A TRACK-TBI Pilot Study
Abstract
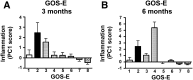
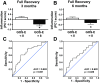
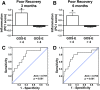
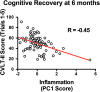
The complex and heterogeneous nature of traumatic brain injury (TBI) has rendered the identification of diagnostic and prognostic biomarkers elusive. A single acute biomarker may not be sufficient to categorize injury severity and/or predict outcome. Using multivariate dimension reduction analyses, we tested the sensitivity and specificity of a multi-analyte panel of proteins as an ensemble biomarker for TBI. Serum was collected within 24 h of injury in a cohort of 130 patients enrolled in the multi-center prospective Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot (TRACK-TBI Pilot) study and run on an array that measured 72 proteins. Using unsupervised principal components analysis, we first identified the subset of protein changes accounting for the most variance across patients. This yielded a group of 21 proteins that reflected an inverse relationship between inflammatory cytokines and regulators of anti-inflammation, and generated an individual inflammatory profile score for each patient. We then tested the association between these scores and computed tomography (CT) findings at hospital admission, as well as their prognostic association with functional recovery at 3 and 6 months (Glasgow Outcome Scale-Extended), and cognitive recovery at 6 months (California Verbal Learning Test, Second Edition) after injury. Inflammatory signatures were significantly increased in patients with positive CT findings, as well as in those who showed poor or incomplete recovery. Inflammation biomarker scores also showed significant sensitivity and specificity as a discriminator of these outcome measures (all areas under the curve [AUCs] >0.62). This proof of concept for the feasibility of multivariate biomarker identification demonstrates the prognostic validity of using a proteomic panel as a potential biomarker for TBI.
Keywords: TBI; biomarkers; proteomics.
Conflict of interest statement
The TRACK-TBI authors declare no competing financial interests in Myriad/RBM including equity, consulting fees, or stock ownership.
Figures




References
-
- Thurman D.J., Alverson C., Browne D., Dunn K.A., Guerrero J., Johnson R., Johnson V., Longlois J.A., Pilkey D., and Sniezek J.E., (1999). Traumatic brain injury in the United States: A report to Congress. Division of Acute Care, Rehabilitation Research, and Disability Prevention, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, US Department of Health and Human Services
-
- McNett M. (2007). A review of the predictive ability of Glasgow Coma Scale scores in head-injured patients. J. Neurosci. Nurs. 39, 68–75 - PubMed
-
- Reith F.C., Synnot A., van den Brande R., Gruen R.L., and Maas A.I. (2017). Factors influencing the reliability of the Glasgow Coma Scale: a systematic review. Neurosurgery 80, 829–839 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

